-
 ⚡️ Reduced Price ⚡️ Take advantage ⚡️ Reduced Price ⚡️ Take advantage
⚡️ Reduced Price ⚡️ Take advantage ⚡️ Reduced Price ⚡️ Take advantage -
-
-

-

Anti-Alcohol Protector - 90 capsules
Anti-Alcohol Protector - 90 capsules
Couldn't load pickup availability
Share
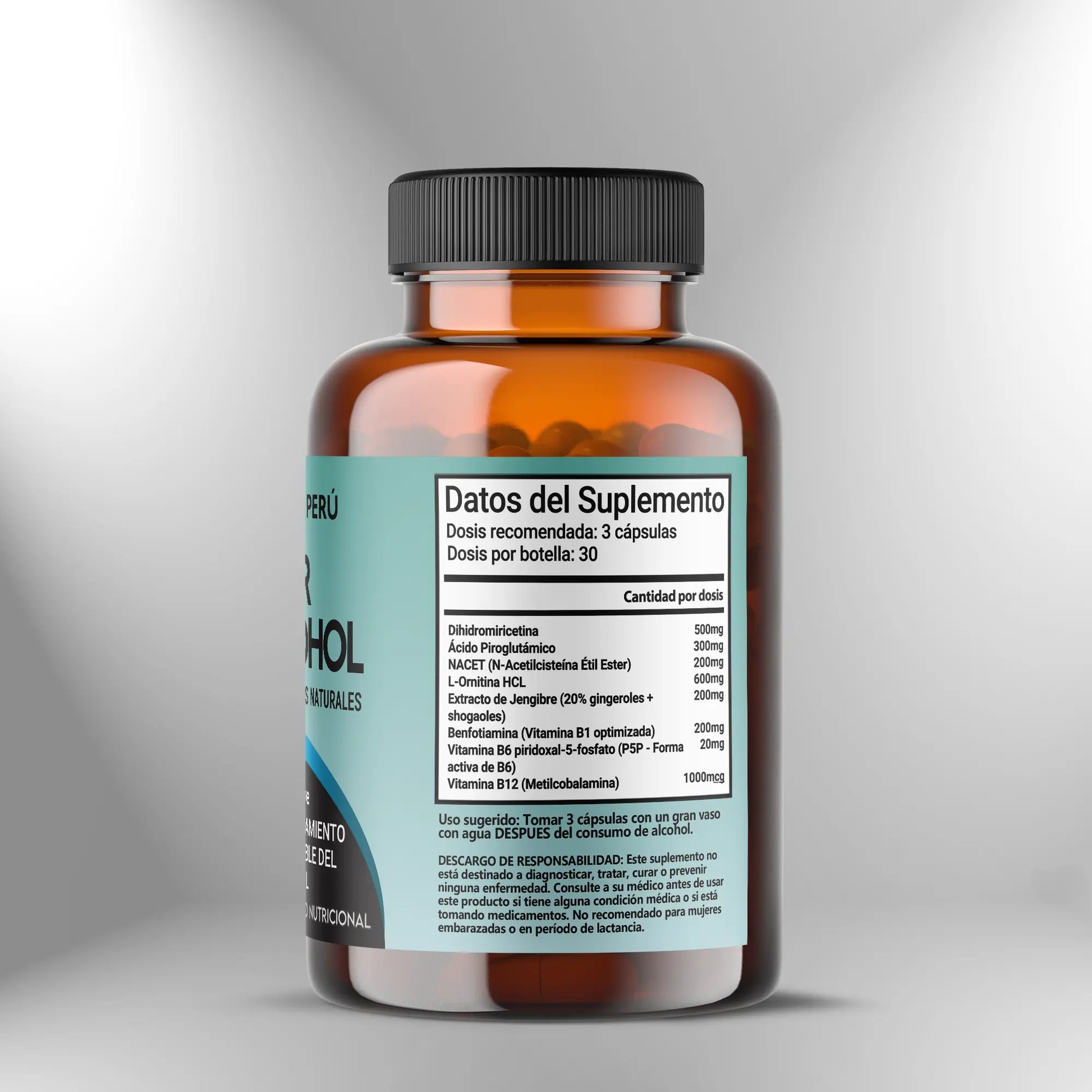
The Anti-Alcohol Protector formula from Nootropics Peru integrates a synergistic complex of bioactive phytochemicals, glutathione precursors, and vitamin cofactors in optimized forms, designed to support the liver's metabolic capacity for acetaldehyde processing by supporting phase I and phase II detoxification systems. This promotes cellular redox homeostasis and the function of aldehyde dehydrogenase and alcohol dehydrogenase enzymes that catalyze the sequential oxidation of ethanol. This formulation contributes to maintaining mitochondrial integrity during oxidative stress associated with alcohol metabolism, supports the urea cycle by providing ornithine, which participates in ammonia conversion, and promotes the neutralization of reactive species generated during ethanol oxidation in hepatocytes through precursors of endogenous antioxidant systems.
Preventive dose - 2 to 3 capsules
For preventive use before alcohol consumption, administer two to three capsules approximately 30 to 60 minutes before consuming alcoholic beverages, allowing time for intestinal absorption of components and distribution to tissues, including the liver, where ethanol metabolism predominantly occurs. Preventative administration ensures that vitamin cofactors are available in active form when enzymes that require them are activated during alcohol metabolism, that glutathione precursors are present in hepatocytes before glutathione consumption increases during acetaldehyde conjugation, and that detoxification enzyme modulators such as dihydromyricetin and ginger extract are in systemic circulation when alcohol exposure begins. The dose of two capsules may be appropriate for moderate alcohol consumption, defined as one to two standard drinks over a period of two to four hours, while three capsules may be more appropriate for more extensive consumption or when the duration of alcohol exposure extends over several hours. The effectiveness of preventive dosing depends on appropriate timing, where very early administration may result in metabolism and elimination of components before alcohol exposure occurs, whereas administration during or after alcohol consumption may not allow the establishment of optimal tissue levels of cofactors and precursors before metabolic demand is increased.
Active support dose - 2 to 3 capsules
During active alcohol consumption lasting several hours, an additional dose of two to three capsules may be administered approximately two to three hours after the initial preventative dose to maintain circulating levels of components that are metabolized and eliminated during prolonged exposure. This stepped-dose strategy is particularly relevant for components with relatively short half-lives, including NACET, which is hydrolyzed by esterases and rapidly metabolized, and water-soluble B vitamins, which are excreted in urine when plasma concentrations exceed renal tubular reabsorption capacity. Administration during alcohol consumption should be with food or at least with plenty of fluids to facilitate swallowing and gastric dilution, reducing the likelihood of gastric discomfort, which can be increased when alcohol and concentrated supplements are present in the stomach simultaneously. Avoid exceeding a total dose of six capsules in a 24-hour period to prevent excessive intake of individual components, particularly B vitamins, where upper tolerable limits, although elevated for most B vitamins, establish a safety ceiling, and to maintain exposure to bioactive components within studied ranges. The decision to administer active support doses should be based on the duration and anticipated amount of alcohol consumption, where brief social events with limited consumption may not require stepped dosing while extended events with sustained consumption may benefit from maintaining component levels through additional administration.
Post-consumer dose - 2 to 3 capsules
After consuming alcohol, administer two to three capsules before bedtime or as soon as practical after the last alcoholic beverage. This promotes the processing of residual alcohol, which continues for several hours after consumption, in a context of optimized cofactor and precursor availability. Post-consumption administration is critical because alcohol metabolism continues while ethanol and acetaldehyde are present in circulation and tissues, a process that can extend for six to eight hours after consumption depending on the amount ingested and individual metabolic rate, which varies substantially between people due to polymorphisms in genes encoding alcohol dehydrogenase and aldehyde dehydrogenase. During the night, while sleep occurs, the liver continues to process alcohol through oxidation, generating acetaldehyde and reactive species that consume glutathione and generate oxidative stress. Therefore, providing glutathione precursors via NACET and cofactors for detoxification enzymes during this period supports continuous processing without interruption by substrate or cofactor depletion. Taking alcohol with a light meal or at least plenty of water is recommended to facilitate absorption and reduce the likelihood of gastric discomfort, although some individuals tolerate it without food, particularly if gastric sensitivity is not a concern. Ensure adequate hydration by drinking at least two to three glasses of water after consumption and before bedtime, as alcohol has diuretic effects by inhibiting antidiuretic hormone, which increases urinary fluid loss. Dehydration contributes to adverse effects associated with alcohol consumption through multiple mechanisms, including increased concentrations of metabolites in the blood and tissues, and impaired cerebral perfusion.
Use as regular liver metabolic support - 2 capsules daily
For individuals who consume alcohol regularly, three or more days per week, taking two capsules daily with a main meal, regardless of the timing of alcohol consumption, provides continuous support for hepatic detoxification capacity and redox homeostasis. This supports the maintenance of glutathione reserves, appropriate expression of detoxification enzymes, and mitochondrial function in hepatocytes, all of which can be compromised during chronic alcohol exposure. This basal dosing approach differs from acute preventive dosing, where the dose is synchronized with a specific alcohol consumption event. Instead, it establishes a sustained supply of cofactors and precursors, optimizing the baseline status of detoxification systems and allowing them to respond more effectively to episodes of alcohol exposure. Administration with a meal containing protein and fat promotes the absorption of lipophilic and chelated components and reduces the likelihood of gastric discomfort that can occur with administration on an empty stomach, particularly during prolonged use. Implement eight- to twelve-week cycles of daily administration followed by seven- to ten-day breaks. These breaks allow for baseline liver function assessment without active supplementation and prevent dependence on exogenous precursors, which could theoretically downregulate endogenous synthesis, although evidence of this phenomenon with components in this formula is limited. During breaks, maintain a balanced diet rich in high-quality protein that provides sulfur-containing amino acids, including cysteine and methionine, which are endogenous precursors of glutathione; cruciferous vegetables, which contain sulfur compounds that induce phase II enzyme expression; and sources of B vitamins, including whole grains, legumes, and animal products.
Strategic timing and acquisition considerations
The absorption and bioavailability of components in this formula are modulated by the presence of food, gastric acidity, and timing in relation to the consumption of other substances that may interfere with absorption or metabolism. Administration with food containing fats favors the absorption of benfotiamine, a lipophilic derivative of thiamine, and may moderate the absorption rate of water-soluble components, reducing peak concentration but extending the duration of absorption and establishing a more sustained pharmacokinetic profile. However, for preventive use where the goal is to achieve high circulating levels rapidly before alcohol exposure, administration without food or with very light food may be preferable to maximize the absorption rate. NACET, as a lipophilic ester of N-acetylcysteine, has improved bioavailability compared to N-acetylcysteine and does not require an empty stomach for proper absorption, although some individuals experience mild nausea with fasting administration, particularly at high doses. Avoid simultaneous use with antacids or proton pump inhibitors that neutralize or reduce gastric acidity, as the acidic environment facilitates capsule disintegration and solubilization of some components. However, the impact on the absorption of components in optimized forms is less than that of simple salts, which require ionization in an acidic environment. Maintain a separation of at least two hours between this formula and high-dose mineral supplements, particularly calcium, magnesium, or zinc, which can form complexes with components, reducing absorption. Also avoid very high-dose fiber supplements, which can adsorb components in the intestinal lumen, reducing mucosal contact for absorption.
Adjustments based on alcohol consumption pattern and individual response
Optimal dosage varies substantially among individuals depending on multiple factors, including the amount and frequency of alcohol consumption, individual metabolic rate determined by genetic polymorphisms in alcohol-metabolizing enzymes and formula components, body mass (which determines volume of distribution), and individual sensitivity to manifestations associated with alcohol consumption. For individuals with a body mass exceeding 90 kilograms or who consume alcohol in amounts exceeding three to four standard drinks, consider increasing the pre-consumption dose to three capsules and the post-consumption dose to three capsules to provide proportionally larger amounts of cofactors and precursors to compensate for the increased volume of distribution and elevated metabolic load. For individuals with known gastric sensitivity or a history of supplement intolerance, start with one capsule during initial use, assessing digestive tolerance, and progress to the standard dose of two to three capsules only if tolerance is appropriate without manifestations of nausea, epigastric discomfort, or changes in bowel movements. If you experience gastric discomfort with the standard dose, administer with substantial food that provides a matrix to buffer direct contact with the mucosa, divide the total dose into two administrations spaced in time, or reduce to two capsules if three cause adverse effects. Individuals who metabolize alcohol rapidly due to genetic variants of alcohol dehydrogenase with increased activity may experience accelerated acetaldehyde accumulation, requiring an emphasis on components that promote aldehyde dehydrogenase activity, including B vitamins, while slow metabolizers may benefit more from components that modulate alcohol dehydrogenase, such as dihydromyricetin. Keep a record of the dose used, timing of administration, amount of alcohol consumed, and perceived response, which provides feedback for optimizing the individual protocol through iterative adjustments based on accumulated experience.
Integration with alcohol impact reduction strategies
Supplementation with an alcohol protector should be integrated as part of a comprehensive approach to minimizing adverse effects associated with alcohol consumption, which includes complementary behavioral and nutritional strategies. Eat food before and during alcohol intake, prioritizing foods containing protein, healthy fats, and complex carbohydrates that slow alcohol absorption, reducing the rate of increase in blood ethanol concentration and allowing the liver to process alcohol more effectively without completely saturating its enzyme capacity. Maintain proper hydration by alternating alcoholic beverages with water in a one-to-one ratio, minimizing dehydration, which contributes to adverse effects, and consuming at least two to three glasses of water before bed and upon waking to facilitate metabolite elimination and rehydration. Limit alcohol consumption to moderate amounts, avoiding excessive consumption that saturates the liver's detoxification capacity regardless of supplementation, leading to acetaldehyde accumulation and oxidative stress that exceeds its protective capacity. Avoid mixing different types of alcoholic beverages, as they may contain congeners—additional compounds generated during fermentation, including methanol, acetone, tannins, and aldehydes—which contribute to adverse effects and require further detoxification. Prioritize quality sleep of appropriate duration after alcohol consumption, recognizing that while alcohol may facilitate sleep onset, it compromises sleep architecture by reducing REM and deep sleep, critical phases for recovery, leading to sleep deficits that contribute to next-day symptoms. Consume a balanced breakfast rich in protein, fruits, and vegetables the following day. This provides amino acids for liver protein regeneration, dietary antioxidants to support endogenous systems, and carbohydrates to restore liver glycogen depleted during alcohol metabolism, which inhibits gluconeogenesis.
Dihydromyricetin (DHM)
Dihydromyricetin (DHM) is a flavonoid extracted from Hovenia dulcis that modulates the activity of alcohol dehydrogenase and aldehyde dehydrogenase, enzymes that catalyze the sequential oxidation of ethanol to acetaldehyde and acetaldehyde to acetate, respectively. This modulation promotes efficient alcohol metabolism and reduces the accumulation of acetaldehyde, a highly reactive metabolite. DHM also modulates GABA-A receptors in the central nervous system through a mechanism that counteracts the effects of ethanol on these receptors, contributing to neurotransmission homeostasis. Preclinical studies suggest that DHM may promote liver protection during ethanol exposure by modulating oxidative stress and the expression of detoxification enzymes, although the precise molecular mechanisms are still being characterized. DHM has moderate bioavailability with significant hepatic metabolism, making dosage and timing of administration important considerations for optimizing its effects.
Pyroglutamic acid
Pyroglutamic acid, also called pidolate or 5-oxoproline, is a cyclic derivative of glutamic acid that participates in the gamma-glutamyl cycle as an intermediate in glutathione metabolism. During glutathione synthesis and degradation, pyroglutamic acid is generated by the cyclization of terminal glutamate from glutathione by gamma-glutamyl cyclotransferase and is converted back to glutamate by 5-oxoprolinase using ATP. Pyroglutamic acid can modulate glutathione homeostasis by affecting the availability of glutamate, which is the rate-limiting precursor for glutathione synthesis when cysteine and glycine are available. The provision of pyroglutamic acid can support the maintenance of glutathione pools during periods of increased demand, such as acetaldehyde metabolism, which consumes glutathione through conjugation catalyzed by glutathione S-transferases. Pyroglutamic acid can also act as an organic osmolyte modulating cell volume and may influence cognitive function through mechanisms including modulation of cholinergic neurotransmission, although evidence in the context of alcohol exposure is limited.
NACET (N-acetylcysteine ethyl ester)
NACET is a lipophilic derivative of N-acetylcysteine where the carboxyl group is esterified with ethanol, increasing its lipophilicity and thus promoting permeability across cell membranes and the blood-brain barrier compared to N-acetylcysteine, which has limited permeability due to the negative charge of its carboxyl group. Once inside cells, the esters are hydrolyzed by esterases, releasing N-acetylcysteine, which provides bioavailable cysteine for glutathione synthesis. Glutathione is a tripeptide composed of glutamate, cysteine, and glycine that acts as the most important endogenous antioxidant and as a cofactor for glutathione peroxidases and glutathione S-transferases. Acetaldehyde metabolism consumes glutathione through conjugation, generating glutathione-acetaldehyde adducts that are excreted, and through neutralization of reactive oxygen species generated during the oxidative metabolism of alcohol. Therefore, the provision of glutathione precursors is relevant during ethanol exposure. NACET can also modulate inflammation through effects on NF-kappaB, which regulates the expression of pro-inflammatory genes, and can promote mitochondrial function by maintaining redox homeostasis, which protects respiratory complexes from oxidative damage.
L-Ornithine HCl
L-Ornithine is a non-proteinogenic amino acid that participates in the urea cycle, a hepatic metabolic pathway that converts toxic ammonia generated during amino acid catabolism into urea, which is excreted by the kidneys. During alcohol metabolism, the generation of acetaldehyde and its oxidation to acetate increase NADH production, which inhibits gluconeogenesis and promotes amino acid catabolism, increasing ammonia generation and causing the urea cycle to operate at increased capacity. Ornithine is a substrate of ornithine transcarbamylase, which catalyzes the condensation of ornithine with carbamoyl phosphate to form citrulline, the first committed step of the urea cycle that occurs in hepatic mitochondria. Providing ornithine can enhance the urea cycle's capacity to process ammonia, particularly during periods of increased generation, although its effectiveness depends on the availability of carbamoyl phosphate and the proper function of subsequent enzymes in the cycle. Ornithine can also modulate the secretion of growth hormone and polyamines involved in cell proliferation and tissue repair, although the relevance of these effects in the context of alcohol exposure requires further characterization.
Ginger extract (20% gingerols + shogaols)
Standardized ginger extract contains gingerols and shogaols, pungent phenolic compounds responsible for the bioactive properties of Zingiber officinale. Gingerols modulate inflammation by inhibiting cyclooxygenases and lipoxygenases, which catalyze the synthesis of pro-inflammatory prostaglandins and leukotrienes, and by inhibiting NF-κB, which regulates the expression of genes encoding cytokines, including TNF-α and IL-6. During alcohol metabolism, the generation of acetaldehyde and reactive oxygen species activates inflammatory signaling pathways in hepatocytes and Kupffer cells, which are resident macrophages in the liver, contributing to hepatic stress. Ginger compounds also modulate gastrointestinal motility by affecting serotonergic and cholinergic receptors, which regulate peristalsis and gastric emptying, thus supporting digestive function that can be compromised during alcohol exposure. Shogaols, which are dehydration products of gingerols during processing or storage, exhibit increased potency in some anti-inflammatory and antioxidant activity assays. Ginger extract also modulates xenobiotic metabolism by affecting the expression of phase II enzymes, including glutathione S-transferases, which conjugate acetaldehyde and other electrophiles, facilitating their elimination.
Benfotiamine (optimized vitamin B1)
Benfotiamine is a synthetic lipophilic derivative of thiamine where the hydroxyl group at position 4 of the pyrimidine ring is substituted with an S-acyl group, increasing lipophilicity and thus promoting intestinal absorption and cellular permeability compared to thiamine hydrochloride, which has limited bioavailability. Once absorbed, benfotiamine is dephosphorylated by intestinal and hepatic phosphatases, releasing thiamine, which is then phosphorylated intracellularly to form thiamine pyrophosphate, the active coenzyme form. Thiamine pyrophosphate is a cofactor for enzymes involved in carbohydrate metabolism, including pyruvate dehydrogenase, which converts pyruvate to acetyl-CoA; alpha-ketoglutarate dehydrogenase in the Krebs cycle; and transketolase in the pentose phosphate pathway, which generates NADPH necessary for the regeneration of reduced glutathione. During alcohol metabolism, the conversion of ethanol to acetaldehyde and acetaldehyde to acetate generates massive amounts of NADH, which inhibit pyruvate dehydrogenase and other NAD+-dependent dehydrogenases, compromising oxidative metabolism and promoting pyruvate accumulation. Thiamine supplementation in the form of benfotiamine can enhance the activity of thiamine-dependent enzymes during metabolic stress associated with alcohol metabolism and may modulate metabolic pathways that generate advanced glycation end products by diverting intermediate metabolites to pathways that do not produce these reactive compounds.
Vitamin B6 (P5P, active form)
Pyridoxal-5-phosphate is the active coenzyme form of vitamin B6, acting as a cofactor for over 140 enzymes that catalyze transamination, decarboxylation, racemization, and other amino acid modifications. During amino acid metabolism, which is increased by alcohol exposure due to the inhibition of gluconeogenesis by elevated NADH levels, thus promoting protein catabolism, pyridoxal-5-phosphate-dependent transaminases catalyze the transfer of amino groups between amino acids and alpha-keto acids. This process involves amino acid interconversion and the generation of intermediates that feed the Krebs cycle. Pyridoxal-5-phosphate is also a cofactor for serine hydroxymethyltransferase, which catalyzes the conversion of serine to glycine, providing methyl groups for purine and thymidylate synthesis, and generating glycine, a precursor to glutathione along with glutamate and cysteine. The enzyme cystathionine beta-synthase, which catalyzes the first step in the transsulfuration of homocysteine to cysteine, requires pyridoxal-5-phosphate. This establishes that vitamin B6 availability influences endogenous cysteine synthesis, which can be limiting for glutathione synthesis when methionine is abundant but dietary cysteine is limited. Pyridoxal-5-phosphate also participates in the synthesis of neurotransmitters, including serotonin, dopamine, and GABA, through aromatic amino acid decarboxylases that convert 5-hydroxytryptophan to serotonin, L-DOPA to dopamine, and glutamate to GABA, modulating neurotransmission that can be altered during alcohol exposure.
Vitamin B12 (methylcobalamin)
Methylcobalamin is a coenzyme form of vitamin B12 that acts as a cofactor for methionine synthase, an enzyme that catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate to homocysteine, regenerating methionine and tetrahydrofolate. This reaction links folate metabolism with methionine and homocysteine metabolism, being critical for the regeneration of tetrahydrofolate, which is necessary for purine synthesis, and thymidylate, required for DNA replication, and for the maintenance of methionine pools, which are precursors to S-adenosylmethionine, a universal methyl group donor in methylation reactions, including the methylation of DNA, proteins, and lipids. During alcohol metabolism, oxidative stress and metabolic disturbances can compromise the function of methionine synthase and the methylation cycle, and homocysteine can accumulate when regeneration to methionine is insufficient or when transsulfuration to cysteine is limited by the availability of pyridoxal-5-phosphate. The provision of methylcobalamin can promote methionine synthase activity by ensuring that the cofactor is available in its active form, although enzyme function also depends on the appropriate redox state of cobalamin, which can be compromised by severe oxidative stress. Adenosylcobalamin, another coenzyme form of B12, acts as a cofactor for methylmalonyl-CoA mutase, which participates in the metabolism of odd-chain fatty acids and branched-chain amino acids. Although methylcobalamin does not participate directly in this reaction, conversion between cobalamin forms can occur intracellularly.
Support for hepatic metabolic detoxification capacity
The synergistic combination of dihydromyricetin, NACET, and B-complex vitamins in optimized forms supports the liver's ability to process ethanol and associated metabolites through coordinated modulation of phase I and phase II enzymes that catalyze the oxidation and conjugation of xenobiotic compounds. Dihydromyricetin modulates the activity of alcohol dehydrogenase and aldehyde dehydrogenase, which catalyze the sequential conversion of ethanol to acetaldehyde and acetaldehyde to acetate, while NACET provides bioavailable cysteine for glutathione synthesis, which acts as a cofactor for glutathione S-transferases that conjugate acetaldehyde and other electrophiles, facilitating their elimination. Benfotiamine provides thiamine pyrophosphate, a cofactor for enzymes involved in the metabolism of acetate generated during alcohol oxidation, including acetyl-CoA synthetase, which converts acetate to acetyl-CoA that can be oxidized in the Krebs cycle or used in lipid and cholesterol synthesis. Ginger extract modulates the expression of phase II enzymes by activating signaling pathways that induce the expression of glutathione S-transferases, UDP-glucuronosyltransferases, and sulfotransferases, increasing the liver's capacity to conjugate reactive metabolites with glutathione, glucuronic acid, or sulfate, generating water-soluble compounds that are excreted in urine or bile. The coordinated provision of vitamin cofactors in bioactive forms ensures that enzymes requiring thiamine pyrophosphate, pyridoxal-5-phosphate, and methylcobalamin operate at optimal capacity during increased metabolic demand associated with alcohol processing, favoring efficient conversion of ethanol to less reactive metabolites and their subsequent elimination via renal and biliary excretion routes.
Optimization of redox homeostasis and neutralization of reactive species
The formulation integrates glutathione precursors, modulators of endogenous antioxidant enzymes, and compounds with direct antioxidant activity, establishing multi-layered protection against oxidative stress generated during alcohol metabolism. NACET provides cysteine, a rate-limiting precursor for glutathione synthesis in hepatocytes, where glutathione consumption is increased during acetaldehyde metabolism through conjugation catalyzed by glutathione S-transferases and through peroxide neutralization by glutathione peroxidases. Meanwhile, pyroglutamic acid participates in the gamma-glutamyl cycle, promoting glutamate regeneration from 5-oxoproline, which can accumulate during accelerated glutathione degradation. Dihydromyricetin exhibits direct antioxidant activity by neutralizing free radicals, including hydroxyl and superoxide radicals, generated during the oxidative metabolism of ethanol by the microsomal ethanol oxidation system involving cytochrome P450 2E1, and by chelating transition metals that catalyze Fenton reactions, generating reactive species. Gingerols and shogaols from ginger extract act as phenolic antioxidants, neutralizing lipoperoxyl radicals that propagate lipid peroxidation in cell membranes, and modulating the expression of endogenous antioxidant enzymes, including superoxide dismutases, catalase, and glutathione peroxidases, by activating Nrf2, a master transcription factor regulating the adaptive response to oxidative stress. B complex vitamins participate in the regeneration of antioxidant capacity by supporting energy metabolism that provides NADPH necessary for the regeneration of reduced glutathione from its oxidized form by glutathione reductase, establishing that optimization of redox homeostasis requires not only neutralization of reactive species but also maintenance of pools of cellular reducing agents that allow continuous cycles of antioxidant protection.
Modulation of hepatic and systemic inflammation
The combination of ginger extract standardized with dihydromyricetin and glutathione precursors supports the modulation of the inflammatory response, which can be activated during alcohol metabolism through the generation of acetaldehyde. Acetaldehyde forms adducts with proteins, generating neoantigens that activate the immune system, and oxidative stress activates pro-inflammatory signaling pathways. Gingerols inhibit cyclooxygenase-2, which catalyzes the synthesis of pro-inflammatory prostaglandins, including PGE2, which mediates vasodilation, increased vascular permeability, and nociceptor sensitization. They also inhibit 5-lipoxygenase, which catalyzes the synthesis of leukotrienes, potent chemoattractants that recruit neutrophils and other leukocytes to sites of inflammation. Inhibition of NF-kappaB by ginger compounds and dihydromyricetin reduces the nuclear translocation of this transcription factor, which regulates the expression of genes encoding pro-inflammatory cytokines, including TNF-alpha, IL-1beta, and IL-6. These cytokines activate Kupffer cells, which are resident macrophages in the liver, and hepatic stellate cells, which can be activated to a fibrogenic phenotype, producing excessive collagen. Glutathione generated from precursors provided by NACET modulates redox-sensitive signaling, including the activation of NF-kappaB and AP-1, transcription factors that respond to changes in cellular redox status. This establishes that maintaining redox homeostasis through glutathione provision has secondary effects on inflammation modulation beyond the direct neutralization of reactive species. The coordinated modulation of multiple points in the inflammatory cascade through inhibition of lipid mediator synthesis, reduction of cytokine expression, and modulation of redox state that controls activation of signaling pathways establishes a comprehensive approach to supporting inflammatory homeostasis during alcohol exposure.
Support for mitochondrial function and energy metabolism
The provision of B-complex vitamin cofactors in bioactive forms that do not require enzymatic conversion supports the function of mitochondrial enzymes that catalyze critical steps in the oxidative metabolism of carbohydrates, lipids, and amino acids, which generates ATP through oxidative phosphorylation. Benfotiamine provides thiamine pyrophosphate, a cofactor for pyruvate dehydrogenase, which catalyzes the oxidative decarboxylation of pyruvate, generating acetyl-CoA that feeds the Krebs cycle; alpha-ketoglutarate dehydrogenase, which catalyzes the rate-limiting step in the Krebs cycle, generating succinyl-CoA and NADH; and the branched-chain alpha-keto acid dehydrogenase complex, which processes leucine, isoleucine, and valine. Pyridoxal-5-phosphate participates in amino acid transamination, generating alpha-keto acids that can feed the Krebs cycle as anaplerotic intermediates, while methylcobalamin participates in propionyl-CoA metabolism, derived from the oxidation of odd-chain fatty acids and amino acids, through the conversion of methylmalonyl-CoA to succinyl-CoA, which enters the Krebs cycle. During alcohol metabolism, the massive generation of NADH from the oxidation of ethanol to acetaldehyde and acetaldehyde to acetate increases the NADH/NAD+ ratio, which inhibits NAD+-dependent dehydrogenases in the Krebs cycle, compromising ATP generation and favoring the diversion of pyruvate to lactate rather than oxidation to acetyl-CoA. This phenomenon can compromise energy metabolism, particularly in tissues with high energy demands, such as the brain and heart. The provision of cofactors that maintain mitochondrial enzyme activity during alcohol-associated metabolic disturbance, combined with antioxidant protection that prevents damage to respiratory complexes by reactive species, supports the maintenance of ATP generation capacity that is critical for optimal cellular function in all tissues.
Promotion of ammonium processing in the urea cycle
The provision of L-ornithine as a substrate for ornithine transcarbamylase, which catalyzes the first committed step of the urea cycle, supports the liver's ability to convert toxic ammonia into urea, a non-toxic, water-soluble compound excreted by the kidneys. This function is particularly relevant during alcohol metabolism, where acetaldehyde generation and NADH accumulation inhibit gluconeogenesis and promote amino acid catabolism by increasing the release of amino groups, which are converted to ammonia via deamination. The urea cycle operates in hepatocytes, where mitochondrial ornithine transcarbamylase condenses ornithine with carbamoyl phosphate to form citrulline. Citrulline is exported to the cytoplasm, where argininosuccinate synthetase condenses citrulline with aspartate to form argininosuccinate. Argininosuccinase cleaves argininosuccinate, generating arginine and fumarate, and arginase hydrolyzes arginine, regenerating ornithine and releasing urea, which is then excreted. Ornithine availability can be limiting for urea cycle rate, particularly when ammonia generation is increased and when endogenous ornithine synthesis from glutamate via the ornithine synthase pathway is insufficient to meet demand. B vitamins participate in amino acid metabolism that feeds the urea cycle through transamination, which transfers amino groups to glutamate, forming alpha-keto acids that can be oxidized or converted to glucose. Glutamate then transfers an amino group to oxaloacetate, forming aspartate, which is a direct substrate for argininosuccinate synthetase. Integrating ornithine provision with cofactors that optimize transamination and amino acid metabolism provides coordinated support for ammonia processing, which can prevent accumulation that compromises central nervous system function, where ammonia interferes with neurotransmission and cerebral energy metabolism.
Modulation of gastrointestinal function and motility
Ginger extract, containing gingerols and shogaols, modulates gastrointestinal tract function by affecting serotonergic receptors, particularly 5-HT3 receptors, which mediate nausea and vomiting when activated in the area postrema of the brainstem, and cholinergic receptors, which modulate intestinal smooth muscle contraction, determining peristalsis and transit time. During alcohol exposure, gastric mucosal irritation from ethanol, an organic solvent that dissolves lipids in cell membranes, delayed gastric emptying, and the accumulation of acetaldehyde, which has direct emetic effects, can compromise digestive function and generate gastrointestinal manifestations, including nausea, bloating, and epigastric discomfort. Ginger compounds promote gastric emptying by stimulating coordinated antral contractions that propel gastric contents into the duodenum and modulating the tone of the pyloric sphincter, which regulates flow from the stomach to the small intestine. The antioxidant and anti-inflammatory activity of gingerols also protects the gastric mucosa from alcohol-induced oxidative damage and inflammation by neutralizing reactive species generated in epithelial cells and inhibiting the production of pro-inflammatory cytokines that can compromise the integrity of the mucosal barrier. The modulation of gastric acid and pepsin secretion by ginger compounds can influence protein digestion and the gastric pH environment, which determines the activity of digestive enzymes, thus affecting multiple aspects of gastrointestinal function that can be compromised during alcohol exposure.
Support for neurotransmitter homeostasis and neurological function
The provision of pyridoxal-5-phosphate and methylcobalamin, which participate in neurotransmitter synthesis and metabolism, supports the maintenance of neurological function during alcohol exposure. Alcohol modulates multiple neurotransmitter systems through effects on GABA, glutamate, serotonin, and dopamine receptors. Pyridoxal-5-phosphate is a cofactor for glutamic acid decarboxylase, which converts glutamate to GABA, the main inhibitory neurotransmitter in the central nervous system that modulates neuronal excitability; for aromatic amino acid decarboxylase, which converts L-DOPA to dopamine and 5-hydroxytryptophan to serotonin; and for serine hydroxymethyltransferase, which generates glycine, an inhibitory neurotransmitter in the spinal cord and brainstem. Methylcobalamin participates in homocysteine metabolism via methionine synthase, which regenerates methionine, a precursor of S-adenosylmethionine. S-adenosylmethionine is a methyl group donor in methylation reactions, including the synthesis of phosphatidylcholine, a major component of neuronal membranes and myelin, and the methylation of neurotransmitters, which modulates their activity and degradation. Dihydromyricetin modulates GABA-A receptors through a mechanism that counteracts the effects of ethanol on these receptors, which normally potentiate GABAergic transmission, generating sedative and anxiolytic effects. This suggests that DHM can modulate some aspects of alcohol's effects on the central nervous system, although the precise mechanisms require further characterization. Maintaining appropriate neurotransmitter synthesis through the provision of vitamin cofactors, combined with antioxidant protection of neurons by glutathione, which neutralizes reactive species that can damage neuronal membranes rich in unsaturated lipids susceptible to peroxidation, provides comprehensive support for neurological function during alcohol-related metabolic disturbances.
Protection of cell membrane integrity and barrier function
The combination of antioxidants that neutralize lipoperoxyl radicals with glutathione precursors and cofactors involved in phospholipid synthesis promotes the maintenance of the structural integrity of cell membranes, which can be compromised during alcohol metabolism through lipid peroxidation initiated by reactive oxygen species. Biological membranes are composed predominantly of phospholipids containing unsaturated fatty acids, particularly at the sn-2 position of glycerol. These carbon-carbon double bonds are susceptible to attack by free radicals, initiating a chain reaction of lipid peroxidation. In this reaction, the generated lipoperoxyl radicals propagate damage to adjacent lipids, generating reactive aldehydes, including malondialdehyde and 4-hydroxynonenal, which form adducts with membrane proteins, compromising their function. Gingerols and shogaols act as phenolic antioxidants that donate hydrogen to lipoperoxyl radicals, terminating the chain reaction, while glutathione generated from NACET acts as a cofactor for glutathione peroxidases that reduce lipid hydroperoxides to less reactive alcohols before they propagate peroxidation. Methylcobalamin participates in the regeneration of methionine, which is a precursor of S-adenosylmethionine that donates methyl groups in a reaction catalyzed by phosphatidylethanolamine N-methyltransferase, converting phosphatidylethanolamine to phosphatidylcholine, establishing that methylation homeostasis influences membrane phospholipid composition. Protecting membrane integrity is particularly relevant in hepatocytes where intense alcohol metabolism generates high oxidative stress, and in mitochondrial membranes where peroxidation can compromise the function of respiratory complexes embedded in the inner mitochondrial membrane, establishing that maintaining membrane integrity is critical for preserving cellular function during alcohol exposure.
Did you know that the human liver can process approximately seven to ten grams of pure ethanol per hour, equivalent to less than one standard drink, establishing a fixed metabolic limit that cannot be significantly accelerated?
The rate of alcohol metabolism is primarily determined by the amount of alcohol dehydrogenase present in hepatocytes, and this amount is relatively constant in non-adapted individuals. Although chronic alcohol consumption can induce expression of cytochrome P450 2E1, which provides an alternative metabolic pathway by slightly increasing overall capacity, the main pathway via alcohol dehydrogenase operates near its maximum capacity even with moderate ethanol concentrations. This means that consuming multiple drinks rapidly leads to an accumulation of ethanol in the blood because intake dramatically exceeds metabolic capacity, and that spacing consumption over several hours allows the metabolism to process alcohol as it is consumed, maintaining lower blood concentrations. The provision of cofactors such as B vitamins does not increase the maximum rate of alcohol dehydrogenase but ensures that the enzyme operates optimally within its inherent kinetic limitations, and that subsequent steps in acetaldehyde metabolism are not limited by cofactor availability.
Did you know that acetaldehyde generated during alcohol metabolism is up to thirty times more reactive than ethanol itself, forming adducts with proteins and DNA that contribute to adverse effects?
Acetaldehyde contains a highly electrophilic aldehyde group that reacts with nucleophilic groups in proteins, including lysine amino groups and cysteine thiol groups, forming covalent bonds that modify protein structure and function. These acetaldehyde-protein adducts can be recognized as neoantigens by the immune system, triggering an inflammatory response, and can compromise the function of critical enzymes when residues in the active site are altered. Acetaldehyde also forms adducts with DNA bases, particularly guanine, causing lesions that require repair by DNA repair systems and that, if not repaired properly, can contribute to mutagenesis. Aldehyde dehydrogenase, which converts acetaldehyde to acetate, is critical for preventing the accumulation of this highly reactive metabolite, and genetic polymorphisms that reduce the activity of this enzyme lead to acetaldehyde accumulation even with moderate alcohol consumption. The provision of glutathione precursors such as NACET promotes acetaldehyde conjugation by glutathione S-transferases that catalyze nucleophilic addition of glutathione to an aldehyde group, providing an additional elimination pathway that complements aldehyde dehydrogenase oxidation.
Did you know that NACET, as a lipophilic ester of N-acetylcysteine, can cross cell membranes up to one hundred times more efficiently than standard N-acetylcysteine due to charge neutralization of the carboxyl group?
At physiological pH, N-acetylcysteine exists predominantly as an anion due to ionization of its carboxyl group. This negative charge prevents permeability across the lipid bilayer of cell membranes, a hydrophobic environment that excludes charged molecules. NACET, through esterification of its carboxyl group with ethanol, neutralizes this charge, generating a molecule that can partition into the lipid environment of membranes and cross them by passive diffusion without requiring transporters. Once inside cells, cytosolic esterases hydrolyze the ester, releasing N-acetylcysteine into the intracellular compartment. There, it can be deacetylated, releasing cysteine for glutathione synthesis, or it can be exported to specific compartments such as mitochondria. This advantage in cellular bioavailability is particularly relevant for cells with limited expression of cysteine or N-acetylcysteine transporters, and for the provision of glutathione precursors to mitochondria where glutathione synthesis occurs locally and where mitochondrial glutathione is critical for respiratory chain protection from oxidative stress generated during oxidative phosphorylation that is increased during alcohol metabolism.
Did you know that during the metabolism of a single alcoholic beverage, the liver can consume more than half of its total glutathione reserves in the conjugation of acetaldehyde and the neutralization of reactive species?
Hepatic glutathione exists in concentrations of five to ten millimolar in hepatocytes, representing a substantial reservoir of antioxidant and conjugation capacity. However, during alcohol metabolism, the generation of acetaldehyde, which is conjugated by glutathione S-transferases, the production of peroxides by the microsomal ethanol oxidation system involving cytochrome P450 2E1, and the generation of radicals during acetaldehyde oxidation by aldehyde dehydrogenase rapidly consume glutathione. De novo glutathione synthesis by glutamate-cysteine ligase and glutathione synthetase requires several hours for complete replenishment of depleted pools, meaning that repeated exposure to alcohol at short intervals can lead to cumulative depletion that compromises protective capacity in subsequent exposures. The provision of glutathione precursors via NACET, which provides cysteine and pyroglutamic acid involved in the gamma-glutamyl cycle, promotes accelerated glutathione synthesis during and after alcohol metabolism. However, the rate of synthesis is still limited by the catalytic capacity of the synthesizing enzymes, which cannot be acutely increased. This critical dependence on glutathione for alcohol processing explains why glutathione depletion due to prior exposure to other xenobiotics, malnutrition limiting precursor availability, or polymorphisms that reduce the expression of glutathione synthesis enzymes increases vulnerability to the adverse effects of alcohol.
Did you know that dihydromyricetin can modulate GABA-A receptors through a mechanism that counteracts some effects of ethanol on these receptors without generating independent sedative effects?
Ethanol potentiates GABAergic transmission by binding to a specific site on GABA-A receptors, increasing the probability and duration of channel opening upon GABA binding. This generates increased chloride influx, which hyperpolarizes neurons and reduces excitability. Dihydromyricetin (DHM) binds to a different site on the GABA-A receptor and modulates the effects of ethanol on the receptor through a mechanism that is not fully characterized but may involve conformational changes that reduce ethanol's ability to potentiate GABAergic transmission. Preclinical studies suggest that DHM reduces some behavioral effects of ethanol, including ataxia and sedation, in animal models when administered before or after alcohol exposure, although translating these effects to humans requires further characterization. Critically, DHM does not activate GABA-A receptors independently, as benzodiazepines or barbiturates do, but rather modulates the effects of ethanol when both are present. This establishes a potentially superior safety profile, given that it does not produce central nervous system depressant effects in the absence of alcohol. This mechanism is distinct from the modulation of alcohol metabolism by DHM and represents an additional effect that may contribute to a modified subjective experience during and after alcohol consumption.
Did you know that alcohol metabolism generates an increased NADH/NAD+ ratio that can reach ten times the normal value in hepatocytes, fundamentally altering multiple metabolic pathways simultaneously?
The oxidation of ethanol to acetaldehyde by alcohol dehydrogenase and of acetaldehyde to acetate by aldehyde dehydrogenase both generate NADH from NAD+. When alcohol metabolism is intense, NADH production exceeds the capacity of the mitochondrial respiratory chain to oxidize NADH back to NAD+. This dramatic increase in the NADH/NAD+ ratio inhibits all NAD+-dependent dehydrogenases that catalyze oxidative reactions, including lactate dehydrogenase, which converts lactate to pyruvate, resulting in lactate accumulation; malate dehydrogenase in the Krebs cycle, which converts malate to oxaloacetate, leading to malate accumulation; and glycerol-3-phosphate dehydrogenase, which participates in the shuttle of reducing equivalents between the cytoplasm and mitochondria. The inhibition of gluconeogenesis by elevated NADH, which inhibits the conversion of lactate to pyruvate and malate to oxaloacetate, promotes hypoglycemia, particularly in fasting individuals. Conversely, the inhibition of beta-oxidation of fatty acids by elevated NADH, which inhibits 3-hydroxyacyl-CoA dehydrogenase, promotes lipid accumulation in hepatocytes. The provision of benfotiamine, which provides thiamine pyrophosphate, favors the diversion of pyruvate toward oxidative decarboxylation by pyruvate dehydrogenase rather than reduction to lactate, although this enzyme is also inhibited by elevated NADH, thus limiting its activity. The regeneration of NAD+ through NADH oxidation in the mitochondrial respiratory chain is critical for restoring metabolic homeostasis, and the protection of mitochondrial function by antioxidants, which prevent damage to respiratory complexes, enhances NADH oxidation capacity.
Did you know that gingerols from ginger can inhibit cyclooxygenase-2 with similar potency to some pharmacological compounds but with a different selectivity profile that minimizes effects on gastric mucosa?
Cyclooxygenase-2 (COX-2) is an inducible isoform of cyclooxygenase that is expressed in response to inflammatory stimuli, including cytokines and oxidative stress. It catalyzes the conversion of arachidonic acid to prostaglandins, including PGE2, which mediates vasodilation, pain, and inflammation. Gingerols inhibit COX-2 catalytic activity through mechanisms that include reduced enzyme expression via effects on NF-κB, which regulates transcription of the gene encoding COX-2, and possibly through effects on enzyme activity, although the precise molecular mechanisms are still being characterized. Unlike synthetic selective COX-2 inhibitors, which can increase cardiovascular risk by inhibiting prostacyclin, a vasodilator and antiplatelet agent produced by the vascular endothelium, gingerols exhibit multiple effects on related pathways, including lipoxygenase inhibition and modulation of thromboxane production, which can balance effects on cardiovascular homeostasis. Gingerols also do not significantly compromise the synthesis of prostaglandins that protect the gastric mucosa, since these are constitutively produced by COX-1 rather than inducible COX-2, establishing a potentially superior gastrointestinal safety profile compared to non-selective cyclooxygenase inhibitors. During alcohol metabolism, where hepatic inflammation can be activated by acetaldehyde and reactive oxygen species, modulation of COX-2 by gingerols may favor a reduction in the production of proinflammatory mediators without compromising the physiological functions of constitutively generated prostaglandins.
Did you know that benfotiamine can achieve intracellular thiamine concentrations up to five times higher than thiamine hydrochloride due to its lipophilicity, which promotes absorption and cellular retention?
Thiamine hydrochloride, the standard form of vitamin B1 supplementation, is a highly polar molecule that requires specific transporters for intestinal absorption and entry into cells. These transporters have a limited capacity, establishing a saturation point where dose increases beyond a certain threshold do not proportionally increase absorption. Benfotiamine, through the substitution of a hydroxyl group with an S-acyl group, increases lipophilicity, allowing absorption by passive diffusion across intestinal membranes without complete dependence on transporters, and increases cell permeability, which facilitates tissue entry. Once absorbed, benfotiamine is converted to thiamine by ester hydrolysis, and the thiamine is then phosphorylated to form thiamine pyrophosphate, the active coenzyme form. The increased intracellular accumulation of thiamine from benfotiamine results in greater availability of thiamine pyrophosphate for enzymes that require it as a cofactor, potentially saturating binding sites and ensuring that enzyme activity is not limited by cofactor availability. During alcohol metabolism, where the demand for thiamine pyrophosphate is increased because thiamine-dependent enzymes, including transketolase, participate in the metabolism of intermediates generated during metabolic disturbance by elevated NADH, providing thiamine in a form that generates high intracellular levels supports the maintenance of appropriate enzyme activity. Benfotiamine may also have additional effects beyond thiamine provision, including activation of transketolase, which diverts glycolytic intermediates to the pentose phosphate pathway, generating NADPH necessary for the regeneration of reduced glutathione.
Did you know that pyroglutamic acid accumulates when glutathione synthesis is inhibited or when glutathione degradation is accelerated, serving as a metabolic indicator of glutathione turnover?
Pyroglutamic acid is generated during the gamma-glutamyl cycle when gamma-glutamyl transpeptidase in the cell membrane cleaves the N-terminal glutamate from glutathione during extracellular degradation, and the resulting cyclic glutamate is converted to pyroglutamic acid by spontaneous cyclization. Pyroglutamic acid must be converted back to glutamate by 5-oxoprolinase, which uses ATP to open the ring and regenerate the open-chain form, completing the cycle that allows glutamate to be reused in the synthesis of new glutathione. When the demand for glutathione is dramatically increased, such as during alcohol metabolism where acetaldehyde conjugation and neutralization of reactive species rapidly consume glutathione, accelerated glutathione degradation generates pyroglutamic acid faster than 5-oxoprolinase can process it, resulting in its accumulation. Alternatively, when glutathione synthesis is inhibited by a deficiency of precursors or by inhibition of gamma-glutamyl-cysteine ligase, the pyroglutamic acid generated during normal glutathione degradation is not efficiently reused because the synthesis of new glutathione is compromised. The provision of exogenous pyroglutamic acid can provide additional substrate for 5-oxoprolinase, facilitating glutamate regeneration, which can then be used in glutathione synthesis. However, effectiveness depends on the availability of cysteine and glycine, which are other necessary precursors, and on the catalytic capacity of the glutathione synthesis enzymes.
Did you know that ornithine participates in the urea cycle, which consumes four high-energy ATP equivalents to convert one molecule of ammonia into urea, making ammonia processing metabolically costly?
The urea cycle is a metabolic pathway that operates primarily in the liver, converting toxic ammonia generated during amino acid catabolism into urea, a non-toxic compound that can be excreted by the kidneys. The cycle requires the condensation of carbamoyl phosphate with ornithine to form citrulline in a step catalyzed by ornithine transcarbamylase; the condensation of citrulline with aspartate to form argininosuccinate, consuming ATP in a step catalyzed by argininosuccinate synthetase; the cleavage of argininosuccinate to arginine and fumarate; and the hydrolysis of arginine to urea and ornithine, regenerating ornithine to begin the cycle anew. The synthesis of carbamoyl phosphate from bicarbonate and ammonia consumes two ATP molecules, while the condensation of citrulline with aspartate consumes an additional ATP molecule, which is cleaved to AMP and pyrophosphate, equivalent to two ATP equivalents. This establishes a total cost of four high-energy ATP molecules per molecule of urea produced. This substantial energy cost illustrates the importance of converting ammonia, which is highly toxic, particularly to the nervous system where it interferes with glutamatergic neurotransmission and energy metabolism, into a compound that can be safely eliminated. During alcohol metabolism, where amino acid catabolism is increased due to the inhibition of gluconeogenesis by elevated NADH, which favors the use of amino acids for energy generation, ammonia production is increased and the urea cycle operates at high capacity. The provision of ornithine can enhance cycle capacity, particularly when ammonia generation exceeds endogenous ornithine synthesis from glutamate, although effectiveness also depends on the availability of carbamoyl phosphate and aspartate, which are other substrates of the cycle.
Did you know that pyridoxal-5-phosphate participates in more than one hundred and forty different enzymatic reactions, more than any other vitamin cofactor, due to the chemical versatility of the aldehyde group of pyridoxal?
Pyridoxal-5-phosphate contains a reactive aldehyde group that forms a Schiff base through condensation with amino groups of amino acids at enzyme active sites, generating an intermediate that is stabilized by resonance with a pyridine ring system and can be manipulated by enzymatic catalysis to promote multiple types of chemical transformations. Transaminases use PLP to transfer amino groups between amino acids and alpha-keto acids through the formation of the pyridoxamine phosphate intermediate; decarboxylases use PLP to stabilize the carbanion generated during carboxyl group removal, allowing the release of CO2; and elimination enzymes use PLP to promote the cleavage of carbon-carbon bonds adjacent to the amino group. This chemical versatility allows PLP to participate in the metabolism of all amino acids, including synthesis, degradation, and interconversion, and in the synthesis of neurotransmitters, including serotonin, dopamine, norepinephrine, GABA, and histamine, through the decarboxylation of amino acid precursors. During alcohol metabolism, where amino acid metabolism is disrupted by elevated NADH, which inhibits gluconeogenesis and favors amino acid catabolism, and where neurotransmitter synthesis may be compromised, providing pyridoxal-5-phosphate in a bioactive form that does not require further phosphorylation ensures that PLP-dependent enzymes function properly. The P5P form is superior to pyridoxine, which requires phosphorylation by pyridoxal kinase, a process that can be limiting. This is particularly relevant in individuals with polymorphisms that reduce the activity of this enzyme, compromising the conversion of pyridoxine to its active form.
Did you know that methylcobalamin participates in methionine synthase, which is the only enzyme in mammals that requires vitamin B12 as a cofactor, along with adenosylcobalamin in methylmalonyl-CoA mutase?
Unlike other B vitamins that act as cofactors in multiple enzymes, vitamin B12 participates in only two reactions in mammals, illustrating its extreme specificity of requirement. Methionine synthase catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate to homocysteine, regenerating methionine and tetrahydrofolate. This reaction is critical for linking folate metabolism to methionine metabolism and for regenerating tetrahydrofolate, which is necessary for the synthesis of purines and thymidylate required for DNA replication. Methylcobalamin at the active site of methionine synthase alternates between methylcobalamin and cob(I)alamine states during catalysis, and cob(I)alamine is highly susceptible to oxidation to cob(II)alamine, its inactive form. The reactivation of methionine synthase when cobalamin is oxidized requires S-adenosylmethionine as a methyl group donor in a reaction catalyzed by the reactivation domain of methionine synthase itself, establishing a cycle where methionine is both a product and a cofactor necessary for maintaining enzymatic activity. During oxidative stress associated with alcohol metabolism, cobalamin oxidation can be increased, compromising methionine synthase activity and resulting in homocysteine accumulation and depletion of 5-methyltetrahydrofolate in a form known as methyl trapping, where folate is trapped in a methylated form that cannot be used in other folate reactions. Providing methylcobalamin can support the maintenance of methionine synthase activity, although its function also depends on cofactor protection against oxidation, which requires appropriate redox homeostasis.
Did you know that ginger extract can modulate gastric emptying through effects on 5-HT3 and muscarinic receptors that regulate gastric antrum motility and pyloric sphincter tone?
Gastric emptying is a coordinated process where gastric contents are propelled from the antrum through the pyloric sphincter into the duodenum by peristaltic contractions regulated by the intrinsic and extrinsic nervous systems and gastrointestinal hormones. Gingerols modulate 5-HT3 serotonergic receptors, which mediate the emetic effects of serotonin released by enterochromaffin cells in the gastrointestinal mucosa. They act as antagonists, reducing the activation of vagal afferent nerves that transmit signals to the vomiting center in the area postrema of the brainstem. Ginger compounds also modulate M3 muscarinic receptors, which mediate gastrointestinal smooth muscle contraction in response to acetylcholine released by neurons in the myenteric plexus. This promotes coordinated contractions that propel contents without generating disorganized contractions that compromise emptying. During alcohol consumption, gastric emptying can be delayed by the direct effects of ethanol on gastric motility and by effects on the central nervous system, which modulates the regulation of gastrointestinal function via the vagus nerve. Delayed gastric emptying prolongs the exposure of the gastric mucosa to alcohol, a direct irritant that dissolves lipids in cell membranes, and can contribute to nausea through gastric distension, which activates mechanoreceptors. Modulation of gastric emptying by ginger extract can promote coordinated propulsion of gastric contents, reducing distension and prolonged mucosal exposure to irritants. However, these effects must be balanced, as very rapid emptying can lead to an abrupt influx of acidic contents into the duodenum, which can cause adverse reactions.
Did you know that during intense alcohol metabolism the liver can divert up to eighty percent of its oxygen consumption to the microsomal ethanol oxidation system involving cytochrome P450 2E1?
The liver, under basal conditions, consumes approximately 20% of total body oxygen despite representing only 2 to 3% of body mass, reflecting a high metabolic rate. During alcohol metabolism, the oxidation of ethanol to acetaldehyde occurs primarily via cytosolic alcohol dehydrogenase, but when ethanol concentrations are high, the microsomal ethanol oxidation system involving cytochrome P450 2E1 in the endoplasmic reticulum contributes substantially. CYP2E1 catalyzes the oxidation of ethanol using NADPH and molecular oxygen, generating acetaldehyde and water, but this process is relatively inefficient, with significant production of reactive oxygen species, including superoxide anion and hydrogen peroxide, as byproducts. The induction of CYP2E1 expression during chronic alcohol consumption increases metabolic capacity but also increases the generation of reactive species that can exceed the capacity of endogenous antioxidant systems to neutralize them, contributing to hepatic oxidative stress. The diversion of oxygen consumption to alcohol metabolism can compromise oxygen availability for other mitochondrial functions, including oxidative phosphorylation that generates ATP, and can create oxygen gradients in the hepatic lobule where the perivenous zone, which normally operates with lower oxygen tension, may experience relative hypoxia. The protection of mitochondrial function by antioxidants that neutralize reactive species generated by CYP2E1 and prevent damage to respiratory complexes supports the maintenance of ATP generation capacity during intensive alcohol metabolism.
Did you know that L-ornithine can be converted into polyamines including putrescine, spermidine, and spermine, which are involved in cell proliferation and tissue repair by stabilizing DNA and RNA?
Ornithine is a substrate of ornithine decarboxylase, which catalyzes the removal of a carboxyl group, generating putrescine, the first polyamine in the synthesis pathway. Putrescine is then modified by the addition of aminopropyl groups derived from decarboxylated S-adenosylmethionine, forming spermidine and spermine. Polyamines are organic cations that bind to negatively charged phosphate groups in DNA, RNA, and proteins, neutralizing charges and stabilizing macromolecular structures. During DNA replication, polyamines stabilize the double helix conformation, facilitating polymerase processivity; during transcription, they stabilize RNA polymerase-DNA complexes; and during translation, they stabilize the structure of ribosomes and transfer RNA, facilitating protein synthesis. Polyamine synthesis is highly regulated by ornithine decarboxylase expression, which is increased during cell proliferation, tissue regeneration, and stress responses. During recovery from alcohol exposure, when hepatocytes may experience stress that compromises cellular integrity, polyamine synthesis can support membrane repair, organelle regeneration, and the synthesis of proteins necessary for restoring function. However, polyamine synthesis consumes S-adenosylmethionine, which is also a methyl group donor in multiple methylation reactions, creating competition between the use of SAM for polyamine synthesis versus methylation reactions. Providing methylcobalamin, which participates in the regeneration of methionine (a precursor of SAM), can help maintain sufficient SAM pools for both functions during periods of increased demand.
Did you know that ginger shogaols are formed by dehydration of gingerols during thermal processing or prolonged storage, and exhibit increased potency in some biological activity assays?
Gingerols contain hydroxyl and ketone groups at specific positions that, under heat or acidic pH conditions, can undergo dehydration through water removal, generating an additional double bond and forming shogaols. This chemical transformation increases conjugation within the molecule and modifies physicochemical properties, including lipophilicity and reactivity. Shogaols exhibit increased anti-inflammatory activity in some models through more potent inhibition of prostaglandin and leukotriene production compared to gingerols, and antioxidant activity that may be superior due to a modified electronic structure that facilitates hydrogen donation to free radicals. Ginger extracts that are thermally processed or derived from dried ginger contain a higher proportion of shogaols compared to fresh ginger, which predominantly contains gingerols, meaning that extract composition influences the biological activity profile. During alcohol metabolism, where modulation of inflammation and neutralization of reactive species are relevant, the presence of both gingerols and shogaols in standardized extract provides a spectrum of bioactive compounds that can act synergistically through multiple mechanisms. The bioavailability of shogaols may differ from gingerols due to differences in lipophilicity and first-pass metabolism, although detailed pharmacokinetic studies in humans are limited, establishing that further characterization of absorption, distribution, and metabolism is necessary for a complete understanding of the relative contribution of different compounds in the extract.
Did you know that glutathione exists in a reduced form with a free thiol group and in an oxidized form as a disulfide-bridged dimer, and that the ratio between these forms reflects the cellular redox state that modulates signaling?
Reduced glutathione contains a cysteine thiol group that can be oxidized by forming a disulfide bridge with another glutathione molecule, generating glutathione disulfide. This reaction occurs during the neutralization of reactive species when glutathione donates electrons. The reduced glutathione/oxidized glutathione ratio in healthy cells is typically greater than 100:1, establishing a reducing environment that maintains protein thiol groups in a functional reduced state and favors reactions requiring a reducing environment. Changes in this ratio toward a more oxidized state modulate the function of redox-sensitive cysteine-containing proteins, including transcription factors whose DNA-binding activity depends on the redox state of cysteines in their binding domain, kinases and phosphatases whose catalytic activity is modulated by cysteine oxidation in their active site, and ion channels whose opening probability is influenced by redox state. During oxidative stress associated with alcohol metabolism, the consumption of reduced glutathione in neutralizing reactive species and conjugating acetaldehyde leads to an accumulation of oxidized glutathione. If this oxidized glutathione is not efficiently regenerated by glutathione reductase using NADPH, the ratio is altered, establishing an oxidative state that can activate stress signaling pathways. Providing glutathione precursors via NACET and pyroglutamic acid promotes the synthesis of new reduced glutathione, increasing the total pool. Providing cofactors involved in NADPH generation, including benfotiamine, which promotes the pentose phosphate pathway for NADPH production, supports the ability of glutathione reductase to regenerate reduced glutathione from its oxidized form, maintaining an appropriate ratio.
Did you know that alcohol inhibits the secretion of antidiuretic hormone by the neurohypophysis through effects on hypothalamic osmoreceptors, generating diuresis that can exceed the volume of fluid consumed in alcoholic beverages?
Antidiuretic hormone, also called vasopressin, is released by the neurohypophysis in response to increases in plasma osmolarity detected by osmoreceptors in the hypothalamus. It acts on renal collecting tubules, increasing the expression of aquaporins, which are water channels in the apical membrane that allow the reabsorption of free water from forming urine back into the bloodstream. Alcohol suppresses antidiuretic hormone secretion through mechanisms that include direct effects on osmoreceptors and possibly through effects on hormone-synthesizing neurons, resulting in reduced aquaporin expression and reduced water reabsorption in collecting tubules. This osmotic diuresis leads to the excretion of urine volume that can exceed the volume of fluid consumed in alcoholic beverages, particularly when the alcohol concentration in the drinks is high, establishing a net negative fluid balance that contributes to dehydration. Dehydration reduces plasma volume, increasing the concentration of alcohol metabolites and other solutes. It also reduces cerebral perfusion, which can contribute to headaches through relative hypoxia of brain tissue and activation of perivascular nociceptors, and compromises renal function in excreting alcohol metabolites and other waste products. Fluid repletion through water intake before, during, and after alcohol consumption is critical for preventing dehydration. Consuming electrolytes, particularly sodium and potassium, which are also excreted in urine during diuresis, helps maintain appropriate fluid and electrolyte balance. Rehydration the following day should consider that complete restoration of fluid balance may require 24 to 48 hours, depending on the severity of dehydration and renal function, which regulates water retention versus excretion.
Did you know that benfotiamine can modulate the hexosamine pathway and the diacylglycerol-protein kinase C pathway, which are activated during hyperglycemia and metabolic stress, by diverting metabolites to the pentose phosphate pathway?
During hyperglycemia or metabolic stress, an increase in the concentration of glycolytic intermediates activates alternative metabolic pathways, including the hexosamine pathway where fructose-6-phosphate is converted to glucosamine-6-phosphate, a precursor of UDP-N-acetylglucosamine used in protein glycosylation, and the diacylglycerol pathway where glyceraldehyde-3-phosphate is reduced to glycerol-3-phosphate, which participates in the synthesis of diacylglycerol that activates protein kinase C. Excessive activation of these pathways can contribute to adverse effects through inappropriate protein glycosylation that alters function, and activation of PKC, which phosphorylates multiple target proteins, disrupting cell signaling. Benfotiamine, by providing thiamine pyrophosphate, activates transketolase, a pentose phosphate pathway enzyme that converts fructose-6-phosphate and glyceraldehyde-3-phosphate into xylulose-5-phosphate and erythrose-4-phosphate. This diverts these intermediates from pathways that generate glycation products and activate PKC to a pathway that generates NADPH and ribose-5-phosphate. This metabolic shift can reduce the accumulation of advanced glycation end products (AGEs), which are formed through the non-enzymatic reaction of sugars with amino groups of proteins. These AGEs cause modifications that alter protein structure and function and are increased during metabolic stress. During alcohol metabolism, where metabolic disturbance due to elevated NADH can lead to the accumulation of glycolytic intermediates, the activation of transketolase by benfotiamine can promote the processing of these intermediates via a pathway that does not generate toxic products. Additionally, NADPH generated via the pentose phosphate pathway is a cofactor of glutathione reductase that regenerates reduced glutathione, establishing that diversion to the pentose phosphate pathway not only reduces activation of pathways that generate adverse products but also promotes antioxidant capacity.
Did you know that NACET can cross the blood-brain barrier more efficiently than N-acetylcysteine, allowing the delivery of glutathione precursors directly to neurons and glial cells?
The blood-brain barrier is a highly selective structure formed by endothelial cells of cerebral capillaries connected by tight junctions that prevent the paracellular passage of molecules. This ensures that only small lipophilic molecules or molecules with specific transporters can enter the brain from the systemic circulation. N-acetylcysteine (NACET) is a negatively charged molecule with limited permeability across the blood-brain barrier, requiring transporters that are expressed at low levels in the cerebral endothelium, thus limiting its entry into brain tissue. NACET, through carboxyl group esterification, increases lipophilicity, allowing partitioning of the lipid bilayer of endothelial membranes and passage via passive diffusion without transporter dependence. Once in brain tissue, NACET is hydrolyzed by esterases present in neurons and glial cells, releasing N-acetylcysteine, which provides cysteine for glutathione synthesis. The brain has a limited capacity for de novo glutathione synthesis and relies partially on importing precursors from circulation, making efficient cysteine delivery via NACET potentially increasing brain glutathione pools. During alcohol consumption, ethanol and acetaldehyde cross the blood-brain barrier and generate oxidative stress in brain tissue through mechanisms similar to those in the liver, and brain glutathione is consumed in neutralizing reactive species. Protecting neurons from oxidative stress is critical because neurons have limited regenerative capacity compared to hepatocytes, and cumulative damage can compromise long-term neurological function. Delivering glutathione precursors via NACET, which efficiently accesses brain tissue, supports neuronal antioxidant capacity, complementing liver protection.
Did you know that different human populations exhibit varying frequencies of polymorphisms in genes that encode alcohol dehydrogenase and aldehyde dehydrogenase, dramatically modulating metabolic capacity and susceptibility to adverse effects of alcohol?
The ADH1B gene, which encodes the beta isoform of alcohol dehydrogenase, has variants, including ADH1B2 , which is common in East Asian populations (frequency 70-80%) and encodes an enzyme with approximately 40 times higher catalytic activity than the ADH1B1 variant, which is predominant in European populations. Individuals with ADH1B2 metabolize ethanol to acetaldehyde much more rapidly, leading to acetaldehyde accumulation that causes facial flushing, tachycardia, and nausea. The ALDH2 gene, which encodes mitochondrial aldehyde dehydrogenase, has the ALDH22 variant, which is also common in East Asian populations (frequency 30-50%) and encodes an enzyme with approximately 90% reduced activity due to the substitution of glutamate for lysine at a position critical for NAD+ binding. Individuals heterozygous for ALDH2*2 have substantially reduced aldehyde dehydrogenase activity, resulting in slow acetaldehyde metabolism and pronounced accumulation that leads to severe adverse effects even with minimal alcohol consumption, while homozygotes have almost no activity, establishing complete intolerance. These genetic variants have been proposed as protective against the development of problematic alcohol use, since unpleasant adverse effects act as a deterrent, illustrating that genetic factors modulate not only metabolism but also behavioral consumption patterns. Understanding individual genotype can inform expectations about metabolic response to alcohol and the potential effectiveness of metabolism-modulating supplementation, since individuals with rapid ethanol metabolism but slow acetaldehyde metabolism may particularly benefit from components that promote aldehyde dehydrogenase activity, such as B vitamins.
Did you know that alcohol compromises intestinal absorption of multiple vitamins and minerals through effects on intestinal mucosal integrity, transporter expression, and pancreatic function?
Chronic alcohol consumption damages the intestinal mucosa through multiple mechanisms, including the generation of reactive oxygen species that cause lipid peroxidation of enterocyte membranes, disruption of tight junctions that increases permeability, allowing translocation of bacteria and bacterial components that trigger inflammation, and impaired epithelial renewal, which normally occurs every three to five days, through reduced proliferation of stem cells in intestinal crypts. This damage compromises the expression and function of nutrient transporters, including transporters of thiamine, folate, vitamin B12 with intrinsic factor, and divalent minerals such as zinc and magnesium, reducing absorption even when dietary intake is adequate. Alcohol also impairs pancreatic function through direct toxic effects on acinar cells that synthesize digestive enzymes, reducing the secretion of lipase, which digests fats; amylase, which digests complex carbohydrates; and proteases, which digest proteins. This compromises macronutrient digestion, resulting in malabsorption of fat-soluble vitamins, which require fat digestion for emulsification and absorption, and of amino acids, which are precursors for the synthesis of glutathione and other compounds. Alcohol also reduces bicarbonate secretion by the pancreas, which neutralizes gastric acidity in the duodenum, establishing an appropriate pH for pancreatic enzyme activity. Therefore, even when enzymes are secreted appropriately, function can be compromised by a suboptimal pH. Supplementation with B-complex vitamins in bioactive forms that do not require enzymatic modification, and provision of glutathione precursors in forms that efficiently cross membranes, such as NACET, can partially compensate for compromised absorption, although optimization of intestinal integrity through reduced alcohol consumption, a balanced diet, and potentially through probiotics that restore a healthy microbiota is necessary for complete correction of malabsorption.
Did you know that ginger compounds can modulate the expression of Nrf2, a master transcription factor that regulates the coordinated expression of more than two hundred genes that encode antioxidant and detoxification enzymes?
Nrf2, nuclear factor erythroid 2-related factor 2, is a transcription factor that, under basal conditions, is sequestered in the cytoplasm by the protein Keap1, which marks Nrf2 for proteasomal degradation, maintaining low levels. During oxidative stress or exposure to electrophiles, sensitive cysteines in Keap1 are modified, altering their conformation and releasing Nrf2, which translocates to the nucleus where it binds to antioxidant response elements in the promoters of target genes, activating transcription. Genes regulated by Nrf2 include superoxide dismutases, which neutralize superoxide radicals; catalase, which neutralizes hydrogen peroxide; glutathione peroxidases and glutathione S-transferases, which neutralize peroxides and conjugate electrophiles; heme oxygenase-1, which degrades heme, generating biliverdin, which has antioxidant activity; and enzymes that synthesize glutathione, including gamma-glutamyl-cysteine ligase, which catalyzes the rate-limiting step. Gingerols and shogaols act as Nrf2 inducers by modifying cysteines in Keap1 or by generating mild reactive species that act as a signal, activating the pathway and establishing a hormetic response where exposure to mild stress induces adaptations that increase resistance to subsequent, greater stress. During alcohol metabolism, where the generation of reactive species can exceed the basal capacity of antioxidant systems, the induction of antioxidant enzyme expression through Nrf2 activation increases overall neutralization capacity. However, gene expression induction requires hours for the synthesis of new proteins, meaning that effects develop gradually rather than immediately. This establishes that Nrf2 activation provides protection that develops over sustained use rather than acute protection during a single episode of alcohol consumption. The combination of Nrf2 activation, which increases endogenous antioxidant capacity, with the provision of glutathione precursors, which increases substrate pools for antioxidant enzymes, establishes a complementary approach where capacity and substrate are optimized simultaneously.
Did you know that alcohol increases intestinal permeability, allowing translocation of bacterial endotoxins from the intestinal lumen to the portal circulation, which activates Kupffer cells in the liver, generating an inflammatory response?
The intestinal barrier is formed by a monolayer of enterocytes connected by tight junctions, which are protein complexes including claudins, occludins, and zonula occludens proteins that seal the space between cells, preventing the paracellular passage of macromolecules, bacteria, and bacterial components from the intestinal lumen into the bloodstream. Alcohol compromises the integrity of tight junctions through multiple mechanisms, including phosphorylation of junction proteins that alters assembly, generation of reactive species that cause oxidative damage to junction proteins, and modulation of the expression of specific claudins that determine junction selectivity. The increased permeability allows translocation of lipopolysaccharide, a component of the outer membrane of Gram-negative bacteria, which acts as a potent immune system activator by binding to the TLR4 receptor on immune cells. Endotoxins that reach the liver via the portal circulation are recognized by Kupffer cells, which are macrophages residing in the hepatic sinusoids. This recognition activates the Kupffer cells to produce pro-inflammatory cytokines, including TNF-alpha, IL-1, and IL-6, which in turn activate hepatic stellate cells, promoting collagen production and generating a systemic acute-phase response. Chronic activation of Kupffer cells during repeated alcohol consumption contributes to persistent hepatic inflammation, which can compromise hepatocyte function and promote fibrogenesis. The anti-inflammatory components in the formula, including gingerols, which inhibit NF-κB, reducing cytokine production, and dihydromyricetin, which modulates the inflammatory response, can attenuate Kupffer cell activation by endotoxins. Additionally, maintaining intestinal barrier integrity through a diet that provides glutamine, which is the preferred fuel of enterocytes, probiotics that modulate the microbiota favoring species that do not massively generate endotoxins, and reducing alcohol consumption, which allows regeneration of the intestinal epithelium, are complementary strategies for reducing endotoxin translocation.
Strategic moment of administration in relation to alcohol consumption
The effectiveness of the Anti-Alcohol Protector depends critically on the timing between administration of the formula and alcohol consumption, since components must reach appropriate circulating and tissue levels before or during the period when ethanol metabolism generates acetaldehyde and reactive species that consume glutathione and activate oxidative stress and inflammatory pathways. For optimal preventive use, administer two to three capsules thirty to sixty minutes before the first alcoholic beverage, allowing sufficient time for intestinal absorption of water-soluble components such as B vitamins, which are rapidly absorbed via specific transporters in the jejunum, and lipophilic components such as benfotiamine and NACET, which require emulsification by bile salts and micelle formation for absorption. If alcohol consumption extends beyond three to four hours, as in prolonged social events, consider administering an additional dose of two capsules approximately halfway through the drinking period to maintain levels of components that are metabolized and eliminated during prolonged exposure. This is particularly relevant for NACET, which is hydrolyzed by tissue esterases, and water-soluble B vitamins, which are excreted when plasma concentrations exceed renal tubular reabsorption capacity. Post-drink administration of two to three capsules before bedtime or as soon as practical after the last alcoholic consumption is critical, given that residual alcohol metabolism continues for six to eight hours while ethanol and acetaldehyde remain in circulation. During this period, the provision of glutathione precursors and enzyme cofactors supports continued processing without substrate depletion. Avoid simultaneous administration with large amounts of high-fat food, which can significantly delay gastric emptying and absorption, although moderate food intake is appropriate to reduce the likelihood of gastric discomfort, particularly in individuals with increased digestive sensitivity.
Conscious moderation of alcohol consumption
Although Alcohol Protector provides metabolic support during the processing of ethanol and its metabolites, the most effective strategy for minimizing adverse effects associated with alcohol is moderating the amount and frequency of consumption within limits that do not completely saturate the liver's detoxification capacity, regardless of supplementation. Limiting consumption to moderate amounts, generally defined as no more than one standard drink per hour, allows the liver's metabolism of alcohol, which proceeds at a relatively constant rate of approximately seven to ten grams of ethanol per hour in the average adult, to process alcohol without massive accumulation of acetaldehyde. However, individual metabolic rates vary substantially due to genetic polymorphisms in alcohol dehydrogenase and aldehyde dehydrogenase, which can increase or decrease the rate of oxidation. Establish an upper limit for total consumption per occasion, taking into account body mass, sex (which influences body water distribution and gastric alcohol dehydrogenase activity, typically lower in women), and individual tolerance observed in previous experiences. Avoid consumption that leads to severe intoxication, where cognitive and motor function is significantly compromised, indicating that blood alcohol concentration exceeds the capacity of neurological systems to compensate for depressant effects on neurotransmission. Alternate alcoholic beverages with water or other non-alcoholic beverages in a one-to-one ratio. This slows the rate of alcohol consumption, allowing time for metabolism, and maintains hydration, since alcohol has diuretic effects by inhibiting antidiuretic hormone, increasing urinary fluid losses that contribute to dehydration and exacerbate adverse effects. Avoid combining alcohol with other central nervous system depressants, including benzodiazepines, opioids, or sedating antihistamines, which potentiate depressant effects through additive or synergistic mechanisms, increasing the risk of respiratory depression. Also avoid combining alcohol with stimulants, including high doses of caffeine, or illicit substances, which mask the perception of intoxication, allowing for excessive alcohol consumption without proper recognition of the level of intoxication. Recognize that supplementation does not completely eliminate the effects of alcohol on judgment, motor coordination, and reaction time, which impair the ability to drive or operate machinery, and that alternative transportation planning is necessary when alcohol consumption occurs.
Nutritional optimization before, during and after alcohol consumption
Strategic eating before, during, and after alcohol consumption modulates ethanol absorption, provides substrates for metabolism and detoxification, and promotes the restoration of metabolic homeostasis. Consume a substantial meal that includes high-quality protein, healthy fats, and complex carbohydrates 30 to 60 minutes before drinking alcohol. This slows gastric emptying and ethanol absorption, reducing the rate of increase in blood alcohol concentration and allowing first-pass hepatic metabolism to process some of the alcohol before it reaches systemic circulation. However, the effect of first-pass metabolism is limited since most alcohol is absorbed in the small intestine, where there is no significant metabolism. Protein provides amino acids, including cysteine, glycine, and glutamate, which are precursors to glutathione, while fats slow gastrointestinal transit, and complex carbohydrates provide glucose, which maintains glycemic homeostasis during the period when alcohol metabolism generates elevated NADH, which inhibits gluconeogenesis, thus promoting hypoglycemia, particularly in individuals who have previously fasted. During alcohol consumption, continue eating, prioritizing nutrient-dense options over processed foods high in empty calories. Include vegetables, which provide dietary antioxidants such as vitamin C, carotenoids, and polyphenols that complement endogenous antioxidant systems; nuts, which provide vitamin E that protects membrane lipids from peroxidation; and fruits, which provide fructose that may slightly modulate ethanol metabolism, although the mechanism and clinical relevance are debated. The day after alcohol consumption, eat a balanced breakfast that includes protein for the regeneration of liver proteins that may be catabolized during alcohol metabolism; carbohydrates to replenish liver glycogen, which is depleted when gluconeogenesis is inhibited; and fruits and vegetables that provide electrolytes, including potassium, which may be depleted by the diuretic effects of alcohol, and antioxidants that continue to neutralize residual reactive species. Consider supplementing with Essential Minerals from Nootropics Peru, which provides a full spectrum of trace and macrominerals, including magnesium, which is a cofactor of enzymes involved in energy metabolism and can be depleted by increased urinary excretion during alcohol consumption; zinc, which is involved in the function of alcohol dehydrogenase and can be mobilized from tissue reserves during intense metabolism; and selenium, which is a component of glutathione peroxidases that neutralize peroxides generated during oxidative stress associated with ethanol metabolism.
• Prioritize high-quality proteins including fish, poultry, eggs, and legumes that provide sulfur-containing amino acids, precursors to glutathione
• Include cruciferous vegetables such as broccoli, kale, and Brussels sprouts, which contain sulforaphane that induces the expression of phase II detoxification enzymes
• Consume sources of vitamin C such as citrus fruits, kiwis, and peppers, which regenerate oxidized vitamin E and act as a cofactor in hydroxylation reactions
• Incorporate choline-rich foods such as eggs and soy, which provide a precursor to phosphatidylcholine, the major phospholipid in hepatocyte membranes.
Strategic hydration and electrolyte replenishment
Proper hydration before, during, and after alcohol consumption is critical for minimizing adverse effects, as alcohol has potent diuretic effects by inhibiting the secretion of antidiuretic hormone by the neurohypophysis. This increases urinary excretion of free water, leading to dehydration, which contributes to headache, fatigue, and impaired cognitive function through reduced plasma volume, decreased cerebral perfusion, and increased concentration of metabolites in tissues. Drink at least two to three glasses of water 30 to 60 minutes before consuming alcohol to establish appropriate hydration. Alternate each alcoholic drink with a similar-sized glass of water during consumption to maintain fluid balance despite increased urinary losses. This approach also slows the rate of alcohol consumption, allowing time for metabolism. Before bed after consuming alcohol, drink at least two to three additional glasses of water to compensate for accumulated losses during the drinking period and throughout the night when insensible losses through respiration and perspiration continue without conscious replenishment. However, avoid excessive consumption, which can lead to frequent urination during the night and compromise sleep quality. Upon waking, rehydrate immediately with at least two glasses of water and continue regular fluid intake throughout the day, aiming for at least two to three liters total, taking into account increased losses from the previous day. Consider including electrolytes in rehydration fluids, particularly sodium and potassium, which are excreted in urine and can be depleted during prolonged alcohol consumption. This can be achieved through consuming broths that provide sodium, coconut water that provides potassium, or oral rehydration solutions containing balanced electrolytes in concentrations optimized for intestinal absorption. Avoid rehydrating solely with beverages containing high amounts of caffeine, such as strong coffee or energy drinks, as caffeine has mild diuretic effects that can exacerbate dehydration. However, moderate amounts of coffee or tea are acceptable when combined with plenty of water. Water quality is particularly relevant in regions where tap water contains contaminants or minerals in concentrations that can compromise digestive function. Filtered or bottled water is preferable, as it provides hydration without the additional burden of compounds that require hepatic processing.
Prioritizing restorative sleep and recovery
Although alcohol can facilitate sleep onset through depressant effects on the central nervous system that reduce sleep latency, it compromises sleep architecture and quality through multiple mechanisms, including suppression of REM sleep (the phase where memory consolidation and emotional processing occur), sleep fragmentation with frequent awakenings during the second half of the night as alcohol is metabolized and its sedative effects diminish, and a reduction in deep, slow-wave sleep (the phase where growth hormone secretion and tissue repair are maximized). Prioritize an appropriate sleep duration of seven to nine hours after alcohol consumption, recognizing that quality may be suboptimal and require extended duration to achieve an equivalent amount of restorative sleep. Establish an optimal sleep environment, including a cool temperature of 18 to 20 degrees Celsius, which promotes sleep onset; complete darkness using blackout curtains or a sleep mask, which prevents melatonin suppression by ambient light; and silence or white noise, which masks disruptive sounds. Avoid using electronic devices that emit blue light during the hour before bedtime, as this suppresses melatonin secretion by affecting intrinsically photosensitive retinal ganglion cells that project to the suprachiasmatic nucleus, regulating the circadian clock. Blue light filters or apps that modify screen color temperature can partially mitigate these effects. If sleep is significantly compromised after alcohol consumption, with frequent awakenings or a feeling of unrefreshing sleep, consider a short nap of 20 to 30 minutes the following day. This provides partial recovery without interfering with the ability to initiate subsequent nighttime sleep. Avoid prolonged naps of more than 60 minutes, as these can lead to sleep inertia, causing grogginess upon waking and compromising nighttime sleep. Recognize that full recovery from sleep deficit and restoration of metabolic homeostasis following significant alcohol consumption may require two to three days of appropriate quality sleep, and that frequent alcohol consumption that chronically compromises sleep generates cumulative sleep debt that impairs cognitive function, emotional regulation, immune function, and metabolism for extended periods even when acute manifestations of alcohol consumption have resolved.
Moderation of physical activity during the recovery period
Although regular physical activity is an important component of a healthy lifestyle that supports cardiovascular function, metabolism, and stress regulation, intense exercise for 24 to 48 hours after significant alcohol consumption can exacerbate dehydration, compromise thermoregulation, and increase metabolic demands in a context where glycemic homeostasis and liver function are still recovering. Limit exercise the day after alcohol consumption to light to moderate activity, including walking, gentle stretching, or yoga, which promotes circulation without generating excessive cardiovascular or metabolic stress. Avoid high-intensity exercise, including high-intensity interval training or heavy weightlifting, which substantially increases heart rate, generates lactate production that can accumulate when lactate metabolism is compromised by elevated residual NADH, and increases the generation of reactive oxygen species in skeletal muscle, which can exacerbate oxidative stress when antioxidant capacity is still recovering. If exercise is performed during a recovery period, ensure increased hydration before, during, and after activity to compensate for losses through sweating in the context of baseline dehydration, consuming at least 500 milliliters of additional water for every 30 minutes of moderate exercise. Monitor heart rate during exercise, noting if it is elevated relative to perceived exertion, which may indicate impaired cardiovascular regulation due to dehydration or electrolyte imbalances. Reduce intensity or discontinue activity if you experience dizziness, severe headache, nausea, or irregular palpitations, which may indicate an inadequate cardiovascular response. Resume normal-intensity training only when hydration is fully restored, adequate sleep quality has been achieved, and cognitive and physical function have returned to baseline levels, typically requiring 48 to 72 hours after significant alcohol consumption, depending on the amount consumed and individual recovery capacity.
Strategic complementarity with synergistic cofactors
The effectiveness of the Anti-Alcohol Protector can be optimized through complementary supplementation with additional cofactors involved in metabolic pathways related to detoxification, antioxidant protection, and liver function. Alpha-lipoic acid is an amphipathic antioxidant that neutralizes reactive species in aqueous and lipid compartments, regenerates oxidized vitamin C and vitamin E, extending antioxidant capacity, and participates as a cofactor in mitochondrial multi-enzyme complexes, including pyruvate dehydrogenase, which converts pyruvate to acetyl-CoA. Since pyruvate dehydrogenase can be compromised during alcohol metabolism due to NADH accumulation, consider supplementation with 300 to 600 milligrams of alpha-lipoic acid daily during periods of regular alcohol consumption. Silymarin extracted from Silybum marianum modulates liver function through antioxidant and anti-inflammatory effects, and potentially by stimulating protein synthesis in hepatocytes, thus promoting regeneration. It can also modulate transporters that mediate hepatic uptake and biliary excretion of compounds. Consider a standardized extract that provides 200 to 400 milligrams of silymarin daily. The Vitamin C Complex with Camu Camu from Nootropics Peru provides ascorbic acid along with bioflavonoids and naturally sourced phytochemicals that act synergistically, regenerating oxidized vitamin E during the neutralization of lipoperoxyl radicals and acting as a cofactor for enzymes involved in collagen synthesis, which maintains the integrity of the hepatic extracellular matrix. Consider 1,000 to 2,000 milligrams distributed throughout the day. Taurine is a conditionally active amino acid that conjugates bile acids, facilitating lipid emulsification. It acts as an osmoregulator, maintaining cell volume, and has cytoprotective effects in hepatocytes by modulating calcium homeostasis and oxidative stress. Consider one to two grams daily, particularly during periods of frequent alcohol consumption. L-carnitine transports long-chain fatty acids to mitochondria for beta-oxidation and can act as an acyl buffer, preventing the accumulation of acyl-CoA, which inhibits metabolic enzymes. Consider 500 to 1,000 milligrams, particularly if lipid metabolism is compromised during chronic alcohol consumption, which promotes lipid accumulation in hepatocytes. Maintain appropriate time separation between different supplements, considering potential interactions. Administer the Anti-Alcohol Protector according to a timing protocol based on alcohol consumption, and take complementary supplements at separate times to optimize absorption and minimize competition for transporters or effects on gastric pH that can modulate bioavailability.
Monitoring of individual response and protocol adjustment
The effectiveness of the Anti-Alcohol Protector and the need for dosage or protocol adjustments varies substantially among individuals due to genetic polymorphisms that affect alcohol metabolism and formula components, body mass that determines volume of distribution, baseline liver function that determines detoxification capacity, and lifestyle factors including diet, hydration, and sleep that modulate recovery. Establish a baseline before initiating formula use by documenting typical manifestations experienced after alcohol consumption, including headache intensity, fatigue, cognitive impairment, gastrointestinal symptoms, and total recovery period duration, providing a reference for evaluating supplementation effectiveness. During initial formula use, experiment with administration timing, including preventive dosing 30 versus 60 minutes before consumption, including or excluding doses during consumption, and post-consumption dosing immediately after the last drink versus before bedtime, identifying the protocol that generates the best response based on individual experience. Observe whether a dosage of two versus three capsules per administration results in differences in effectiveness. Consider increasing to three capsules if two is insufficient or if alcohol consumption is high, or maintaining two capsules if effectiveness is appropriate while minimizing component intake. Document any adverse reactions, including gastric discomfort, nausea, or changes in bowel movements, which may indicate sensitivity to specific components or excessive dosage. Adjust by reducing the dose, administering with more substantial food, or dividing the dose into more spaced-out administrations. Evaluate effectiveness not only based on the intensity of immediate symptoms but also on recovery of cognitive and physical function, sleep quality after consumption, and the ability to resume normal activities the following day. Recognize that goals may vary among individuals, with some prioritizing headache minimization while others prioritize cognitive function or exercise capacity. Recognize that effectiveness may vary depending on the type of alcoholic beverage consumed, as different drinks contain varying congeners—additional compounds generated during fermentation that contribute to adverse effects—and depending on the consumption context, including the presence or absence of food, rate of consumption, and environmental factors such as temperature and altitude, which modulate alcohol metabolism and effects. Maintain flexibility in your protocol, adjusting based on accumulated experience rather than rigidly adhering to a specific dosage, and recognize that while protocol optimization can improve your experience, no supplementation completely eliminates the effects of alcohol or compensates for excessive consumption that exceeds the liver's detoxification capacity.
Experience during active alcohol consumption
During preventive use, where the formula is administered 30 to 60 minutes before alcohol consumption, some individuals report modulation of subjective experience. This may include a smoother transition between sobriety and intoxication, reflecting the effects of dihydromyricetin on GABA-A receptors, and a reduction in the intensity of gastrointestinal symptoms, including nausea or epigastric discomfort, that can occur during consumption, particularly when alcohol is consumed without food. This reduction is attributable to the effects of ginger extract on gastric motility and serotonergic receptors that mediate nausea. The provision of vitamin cofactors and glutathione precursors before alcohol exposure establishes that detoxification systems operate with optimized substrate availability when ethanol metabolism begins. However, changes during active consumption are subtle, given that alcohol metabolism generates a substantial metabolic load that cannot be fully offset by supplementation. Appropriate hydration, with alternating water intake between alcoholic beverages and consuming food that slows ethanol absorption, are complementary strategies that modulate the experience during consumption more significantly than supplementation alone. Some individuals may not perceive obvious differences during active consumption but experience more noticeable changes during the recovery period the following day, establishing that timing of effect assessment is relevant.
Recovery during the first few hours after consumption
For six to twelve hours after the last alcohol consumption, particularly during the night when sleep occurs, residual metabolism of ethanol and acetaldehyde continues, while the post-consumption formula provides processing support. Some individuals report less compromised sleep quality compared to previous experiences without supplementation, potentially reflecting modulation of sleep architecture through the combined effects of magnesium in forms that cross the blood-brain barrier, promoting relaxation, and protection of neuronal function by glutathione generated from NACET, which neutralizes reactive species in brain tissue. Upon waking, common manifestations associated with alcohol consumption, including headache (the intensity of which can be modulated by optimized hydration and reduced inflammation from gingerols), fatigue (which may be less pronounced when mitochondrial function is protected by antioxidants), and cognitive impairment (including difficulty concentrating or reduced processing speed), may vary in intensity depending on the amount of alcohol consumed and individual factors. Experience is highly variable among individuals, reflecting genetic polymorphisms in enzymes that metabolize alcohol and formula components, body mass, baseline liver function, and adherence to complementary strategies including appropriate hydration and nutrition.
Adaptation during the first weeks of regular use
For individuals who consume alcohol three or more days per week and use this formula as hepatic metabolic support by taking two capsules daily, regardless of the timing of alcohol consumption, adaptation during the first two to four weeks may include subtle changes in basal energy and digestive function as redox homeostasis is optimized and detoxification enzyme expression is modulated by Nrf2 activation from ginger extract. Some individuals experience transient changes in stool consistency or frequency during the first week, reflecting electrolyte modulation of intestinal motility and microbiota adaptation; these manifestations typically normalize with continued use. Digestive tolerance is generally good, as chelated and bioactive forms of components exhibit improved absorption with less gastric irritation. However, administration with food is recommended to minimize the likelihood of discomfort, particularly in individuals with gastric sensitivity. During this adaptive phase, establishing a consistent administration routine by integrating it with daily activities, such as preparing morning coffee or main meals, promotes adherence, which is critical for effectiveness. The response assessment should consider not only the intensity of manifestations after alcohol consumption but also parameters such as sleep quality, daytime energy, and ability to maintain normal activities the day after consumption.
Consolidation of effects with sustained use (4-8 weeks)
During the second and third months of consistent use, consolidation of effects occurs through complete replenishment of tissue stores of vitamin cofactors that may be depleted during regular alcohol consumption, sustained expression of antioxidant and detoxification enzymes induced by Nrf2 activation, and metabolic adaptations that optimize alcohol processing. Some individuals report that recovery capacity after alcohol consumption gradually improves, with a reduced duration of adverse effects and less severe functional impairment, reflecting optimized metabolic homeostasis that allows for more efficient processing of alcohol and its metabolites. Baseline liver function may be supported by continuous protection against oxidative stress and modulation of inflammation, although objective assessment using biochemical markers requires appropriate analyses that are beyond the scope of self-assessment. During this phase, some individuals find they can tolerate similar amounts of alcohol with fewer adverse effects, although this should not be interpreted as a license to increase consumption, given that cumulative alcohol damage occurs regardless of the presence or absence of acute symptoms, and that the goal of supplementation is to support metabolic homeostasis rather than facilitate increased consumption. Integrating supplementation with mindful moderation, proper hydration, and a balanced diet establishes a comprehensive approach where supplementation complements, but does not replace, fundamental behavioral strategies.
Maintenance and long-term use (3-6 months)
After three to six months of consistent use, with cycles of eight to twelve weeks of administration followed by seven- to ten-day breaks, the established adaptations are maintained, resulting in optimized metabolic homeostasis that persists during these breaks. This reflects that improvements do not depend solely on continuous exogenous supplementation but also include sustained adaptations in gene expression and cellular function. During these breaks, monitoring parameters such as energy levels, sleep quality, and recovery after alcohol consumption provides feedback on whether improvements depend on active supplementation versus the establishment of independent adaptations, informing decisions about continued use. Long-term use is generally appropriate when alcohol consumption is regular and when perceived benefits justify continued use, although periodic reassessment of the need is prudent, considering that the ultimate goal should be the optimization of function through a combination of supplementation, moderate alcohol consumption, and healthy lifestyle habits. Some individuals use the formula exclusively as a preventative measure and post-consumption strategy during specific alcohol-consuming events rather than as continuous daily supplementation. This approach is valid, although the effects on enzyme expression and tissue replenishment are less pronounced compared to sustained use. Monitoring liver function through periodic biochemical analyses may be prudent for individuals with frequent alcohol consumption, regardless of supplementation, providing an objective assessment of liver status that complements subjective observation of symptoms.
Limitations and realistic expectations
The effectiveness of the Anti-Alcohol Protector is fundamentally limited by the amount and frequency of alcohol consumption. No supplementation can fully compensate for excessive consumption, which saturates the liver's detoxification capacity, leading to acetaldehyde accumulation and oxidative stress that exceeds neutralization capacity, regardless of cofactor provision. Individual variability in response to supplementation is substantial, reflecting genetic polymorphisms that affect alcohol metabolism. Individuals with aldehyde dehydrogenase variants with reduced activity experience pronounced acetaldehyde accumulation, resulting in severe manifestations that can only be partially modulated by supplementation. These polymorphisms also affect the absorption and metabolism of formula components. Lifestyle factors, including diet quality that provides endogenous glutathione precursors and vitamin cofactors from dietary sources, hydration that facilitates metabolite excretion and maintains tissue perfusion, adequate sleep quality that allows for cell recovery and regeneration, and stress management that modulates immune function and the inflammatory response, significantly influence the effectiveness of supplementation, establishing that a comprehensive approach is necessary. This formula should not be interpreted as a treatment for conditions associated with alcohol consumption or as a strategy to facilitate increased consumption, but rather as metabolic support that complements conscious moderation and healthy habits. Expectations should be realistic, recognizing that supplementation optimizes basal metabolic capacity but does not completely eliminate the effects of alcohol on multiple physiological systems.
Initial adaptation phase
During the first three to seven days of use, the body implements homeostatic adjustments, adapting to the increased supply of vitamin cofactors, glutathione precursors, and bioactive compounds. Some individuals experience transient changes in digestive function, including slight modifications in stool consistency or frequency, reflecting modulation of intestinal motility by ginger extract and adjustments in the gut microbiota to formula components. These manifestations typically resolve during the second week of use. Administration with substantial food, including protein and fat, promotes digestive tolerance, reducing the likelihood of mild nausea or epigastric discomfort that may occur with fasting, particularly during the adaptation phase. If you experience persistent gastric discomfort, consider temporarily reducing to one capsule daily for an additional week to allow for gradual adaptation before progressing to the standard dose, or dividing the daily dose into widely spaced administrations to maximize time separation. Some individuals may experience subtle changes in energy or alertness during the first few days, reflecting optimization of energy metabolism by vitamin cofactors, although these changes are typically subtle rather than dramatic. Manifestations that are severe, persist beyond two weeks, or include signs of hypersensitivity such as hives, facial swelling, or difficulty breathing require immediate discontinuation and appropriate evaluation, although such reactions are rare with components in this formula.
Commitment required for optimal results
The effectiveness of the Anti-Alcohol Protector depends critically on consistent administration and adherence to a timing protocol that synchronizes supplementation with alcohol consumption to optimize cofactor availability when metabolic demand is increased. For preventive and post-consumption use during specific alcohol consumption events, administering two to three capsules thirty to sixty minutes before the first drink and two to three capsules before bedtime after the last drink establishes a minimum protocol, with the possibility of additional doses during prolonged consumption lasting more than four hours. For use as hepatic metabolic support in the context of regular alcohol consumption, administering two capsules daily with the main meal for cycles of eight to twelve weeks followed by breaks of seven to ten days establishes a pattern that optimizes replenishment of tissue reserves and expression of detoxification enzymes while preventing excessive accumulation. Integrating supplementation with key behavioral strategies, including moderating alcohol consumption to no more than one standard drink per hour, alternating alcoholic beverages with water in a one-to-one ratio, eating food before and during alcohol intake, and prioritizing adequate sleep after consumption, is essential for maximizing effectiveness. This approach also includes monitoring response by observing parameters such as the intensity and duration of post-consumption symptoms, sleep quality, energy levels, and cognitive function the following day. This provides feedback for protocol adjustments and individual effectiveness assessments, guiding decisions about continued or modified use.
Specific combination of ingredients
The Anti-Alcohol Protector formula has been designed with a unique combination of highly bioavailable ingredients, optimized to ensure maximum absorption and effectiveness. The integration of ingredients such as dihydromyricetin (DHM) , NACET , L-Ornithine HCl , and ginger extract , in the correct doses, allows the body to receive the necessary support to protect itself and recover from the stress caused by alcohol consumption. The precise ratio of each ingredient ensures synergistic effects, enhancing the benefits for faster and more effective action.
Superior effectiveness
What makes Anti-Alcohol Protector special is its ability to provide faster and longer-lasting results compared to traditional formulas. The precise combination of its ingredients not only reduces the immediate effects of alcohol but also supports long-term cell regeneration and liver function. The synergy between the formula's components works together to accelerate detoxification and promote optimal recovery, resulting in less discomfort after alcohol consumption. Furthermore, the formula offers a significant advantage over others on the market, which often focus on a single ingredient or limited effects.
Specific benefits
This complex has been developed to address specific problems associated with alcohol consumption, such as stomach upset, fatigue, and dehydration. Offering multiple benefits in one, Anti-Alcohol Protector not only combats the immediate effects of alcohol intake but also supports liver function and cell regeneration, optimizing the body's detoxification process. The combination of B vitamins , antioxidants , and peptides helps improve tolerance and reduce cell damage, ensuring results backed by the synergy of scientifically proven ingredients.
User convenience
The dosage of Anti-Alcohol Protector is practical and easy to follow: simply take 3 capsules with water after consuming alcohol, making it easy to incorporate into your daily routine without complications. The formula is designed to be well-tolerated by most people, reducing the risk of side effects common with other solutions. Thanks to its capsule format and optimized dosage, this product provides a convenient way to effectively address the negative effects of alcohol without having to worry about complicated dosing regimens or strict consumption times.
Nutritional optimization
To enhance the effects of Anti-Alcohol Protector , it's important to follow a balanced, nutrient-rich diet that promotes the absorption and benefits of the formula. Nutrients such as magnesium, zinc, and vitamin C are essential for the optimal functioning of the antioxidants in the formula, helping to boost detoxification and cellular repair. Consuming foods rich in these vitamins and minerals, such as fruits, leafy green vegetables, nuts, and seeds, will effectively support absorption and maximize results. It's also recommended to avoid excessive consumption of processed foods, trans fats, and refined sugar, as these can interfere with the supplement's benefits.
Lifestyle habits
Lifestyle plays a fundamental role in the effectiveness of Anti-Alcohol Protector . Maintaining adequate sleep patterns, with at least 7-8 hours of restorative rest, is key to supporting cell regeneration and optimizing the body's response to the supplement. Managing stress through techniques such as meditation or yoga also improves effectiveness, as it reduces inflammation and supports overall health. The importance of rest cannot be underestimated, as a rested and balanced body will respond better to the treatment. Establishing healthy routines to improve both sleep and stress management will significantly increase the formula's effectiveness.
Physical activity
Engaging in moderate to intense physical activity, such as walking, running, or weight training, helps accelerate the recovery process, improves blood circulation, and optimizes metabolism, thus supporting the effectiveness of the Anti-Alcohol Protector . A frequency of 3-5 times per week is recommended for best results. During workouts, supplementing with cardiovascular and strength exercises will help maximize the formula's impact on overall health. Taking the supplement before or after workouts can also help improve alcohol tolerance and speed up the body's recovery after consumption.
Hydration
Proper hydration is essential to enhance the effects of Anti-Alcohol Protector . It is recommended to consume at least 2-3 liters of water per day, depending on individual needs and activity level. High-quality, contaminant-free water is crucial for efficient hydration and improved absorption of the formula's nutrients. During and after alcohol consumption, maintaining good hydration is even more important, as it helps reduce dehydration and improves liver function. Strategies such as consuming electrolyte-enhanced water can also be beneficial for maintaining fluid balance and optimizing the formula's effects.
Supplementation cycle
Consistency is key to getting the best results from Alcohol Protector . Following the recommended dosage protocol and taking the supplement at the indicated times will maximize absorption and long-term benefits. Avoiding common mistakes, such as skipping doses or altering the timing of consumption, is crucial to maintaining the supplement's effectiveness. It is important to follow the protocol regularly to obtain the full effects, and it is also recommended to continue supplementation for longer periods to improve tolerance and protection against the effects of alcohol.
Metabolic factors
Optimizing metabolism is essential to maximizing the effectiveness of Anti-Alcohol Protector . Maintaining proper hormonal balance and reducing inflammation levels will allow for greater cellular sensitivity and faster recovery. This can be achieved through a diet rich in antioxidants, regular exercise, and stress management. By improving metabolism and cellular function, the body will respond more efficiently to the supplement, leading to more effective protection against the harmful effects of alcohol.
Synergistic complements
To further enhance the effects of Anti-Alcohol Protector , certain synergistic cofactors and supplements can be considered. For example, magnesium and vitamin C are supplements that can improve the formula's bioavailability, aiding in detoxification and cellular recovery. Other supplements, such as alpha-lipoic acid or silymarin (milk thistle extract), can be beneficial in supporting liver health and increasing the body's detoxification capacity. These combinations amplify the supplement's effects and can improve the body's response to alcohol consumption.
Mental aspects
Mindset and expectations play a crucial role in the effectiveness of Anti-Alcohol Protector . Maintaining a positive attitude and managing stress effectively are factors that can enhance the supplement's results. Practicing mindfulness or relaxation techniques can also reduce the negative effects of alcohol on mental and physical health. By focusing on a healthy lifestyle and adopting a balanced approach to alcohol tolerance, both the physical and psychological results of the supplement are improved.
Personalization
Every body responds differently, so it's important to tailor the use of Alcohol Protector to your individual needs. Listening to your body's signals, adjusting the dosage based on your response, and being flexible with the protocol can maximize the benefits. Each person may experience the supplement's effects at a different pace, so it's important to be patient and adjust your approach as needed, while keeping your long-term goals in mind.
Support for liver detoxification and acetaldehyde metabolism
• Alpha-lipoic acid : An amphipathic antioxidant that crosses aqueous and lipid compartments, neutralizing reactive species generated during ethanol metabolism by cytochrome P450 2E1. It acts as a cofactor for mitochondrial multi-enzyme complexes, including pyruvate dehydrogenase, which converts pyruvate to acetyl-CoA during the processing of acetate generated from acetaldehyde oxidation. Alpha-lipoic acid regenerates oxidized vitamin C and vitamin E, extending the antioxidant capacity established by dihydromyricetin and ginger components. It also participates in the regeneration of glutathione from its oxidized form by modulating the expression of gamma-glutamylcysteine ligase, which catalyzes the rate-limiting step in glutathione synthesis. This synergy with NACET, which provides cysteine as the rate-limiting precursor, while alpha-lipoic acid promotes the expression of the enzyme machinery involved in glutathione synthesis.
• Silymarin : A flavonoid extracted from Silybum marianum that modulates hepatocyte function through antioxidant effects that neutralize reactive species generated during alcohol metabolism, anti-inflammatory effects by inhibiting NF-kappaB, which reduces the expression of pro-inflammatory cytokines in Kupffer cells activated by endotoxins translocated from the intestine, and potentially by stimulating protein synthesis in hepatocytes, thus promoting regeneration. Silymarin modulates transporters that mediate hepatic uptake and biliary excretion of compounds, influencing the pharmacokinetics of alcohol metabolites, and acts synergistically with ginger extract, which also inhibits NF-kappaB, establishing dual modulation of inflammatory signaling pathways through complementary mechanisms.
• Taurine : A conditionally active amino acid that conjugates bile acids, facilitating lipid emulsification and promoting the absorption of lipophilic components of the formula, including benfotiamine and NACET. It acts as an osmoregulator, maintaining cell volume during osmotic stress generated by the accumulation of alcohol metabolites, and has cytoprotective effects in hepatocytes by modulating calcium homeostasis, preventing the activation of apoptotic pathways, and by neutralizing hypochlorite generated by myeloperoxidase during the activation of neutrophils that infiltrate the liver during the inflammatory response. Taurine complements the effects of L-ornithine in ammonia processing, since both amino acids participate in nitrogen homeostasis, providing dual support to liver function during the increased metabolic demand caused by alcohol metabolism.
• Vitamin C Complex with Camu Camu : Provides ascorbic acid along with bioflavonoids and naturally sourced phytochemicals that act synergistically, regenerating oxidized vitamin E by neutralizing lipoperoxyl radicals that propagate lipid peroxidation in hepatocyte membranes. It acts as a cofactor for enzymes involved in collagen synthesis, which maintains the integrity of the hepatic extracellular matrix, and promotes the absorption of formula components by affecting gastric pH and modulating intestinal transporters. Vitamin C recycles oxidized dihydromyricetin during antioxidant activity, extending its functional half-life, and provides electrons for glutathione regeneration from its oxidized form through non-enzymatic reduction, establishing complementarity with the provision of precursors by NACET and pyroglutamic acid.
Optimization of redox homeostasis and antioxidant protection
• Vitamin D3 + K2 : Vitamin D3 modulates the expression of genes encoding antioxidant enzymes, including glutathione peroxidases and superoxide dismutases, by affecting the vitamin D receptor, which acts as a transcription factor. It works synergistically with ginger extract, which activates Nrf2, also regulating antioxidant enzyme expression through a complementary mechanism. Vitamin K2 participates in the carboxylation of proteins containing gamma-carboxyglutamic acid, including matrix proteins that prevent soft tissue calcification, and modulates mitochondrial function by affecting the respiratory chain, which can reduce the generation of reactive species during oxidative phosphorylation. The combination with a formula containing glutathione precursors and direct antioxidants establishes multi-layered protection where enzyme expression, cofactor provision, and direct neutralization are optimized in a coordinated manner.
• CoQ10 + PQQ : Coenzyme Q10 acts as an electron carrier in the mitochondrial respiratory chain, transferring electrons from complexes I and II to complex III. In its reduced form, ubiquinol neutralizes lipoperoxyl radicals in mitochondrial membranes, preventing lipid peroxidation that compromises the function of respiratory complexes during oxidative stress generated by alcohol metabolism. Pyrroloquinoline quinone acts as a cyclic redox cofactor that can accept and donate electrons multiple times without being consumed. It stimulates mitochondrial biogenesis by activating PGC-1α, which coordinates the expression of nuclear and mitochondrial genes that encode mitochondrial proteins. This combination synergizes with benfotiamine, which provides thiamine pyrophosphate for mitochondrial enzymes, and with NACET, which provides glutathione for mitochondrial protection, optimizing mitochondrial function by supporting energy generation, providing antioxidant protection, and promoting biogenesis.
• Methylfolate : A bioactive form of folate that participates with methylcobalamin in methionine synthase, which regenerates methionine from homocysteine. This establishes a direct synergy since both cofactors are required by the same enzyme, where methylcobalamin is a prosthetic cofactor and methylfolate is a methyl group donor. Methionine regeneration maintains pools of S-adenosylmethionine, a universal methyl group donor in methylation reactions, including the synthesis of phosphatidylcholine, a major phospholipid in cell membranes whose integrity is compromised during alcohol metabolism, and DNA methylation, which regulates gene expression. Methylfolate also regenerates tetrahydrofolate, which participates in the synthesis of purines and thymidylate, both necessary for DNA replication during hepatocyte regeneration. Therefore, methylfolate complements methylcobalamin not only in the specific methionine synthase reaction but also in providing intermediates for multiple pathways that require one-carbon units.
Support for gastrointestinal function and absorption
• L-Glutamine : An amino acid that is the preferred fuel of enterocytes, providing energy for maintaining epithelial turnover, which occurs every three to five days and is critical for intestinal barrier integrity, which is compromised during alcohol consumption. Glutamine participates in glutathione synthesis as a precursor to glutamate, the first amino acid incorporated into the tripeptide. It establishes synergy with NACET, which provides cysteine, and with pyroglutamic acid, which participates in the gamma-glutamyl cycle, optimizing the supply of all the necessary precursors for glutathione synthesis in enterocytes, where protection against oxidative stress is critical for maintaining absorptive function. Glutamine also modulates intestinal permeability by affecting the expression of tight junction proteins, preventing the translocation of endotoxins that activate hepatic inflammation.
• Probiotics of specific strains (Lactobacillus plantarum, Bifidobacterium longum) : These modulate the composition of the gut microbiota, favoring species that do not massively produce endotoxins and that produce short-chain fatty acids, including butyrate, which is fuel for colonocytes and has anti-inflammatory effects by inhibiting NF-kappaB. The probiotics improve intestinal barrier integrity by producing metabolites that strengthen tight junctions, reducing lipopolysaccharide translocation, which activates Kupffer cells. This synergy with anti-inflammatory components of the formula, including gingerols, which act in the liver while the probiotics act in the intestine, establishing coordinated protection. The probiotics can also metabolize components of the formula, modifying bioavailability through deconjugation or conversion to active metabolites, although specific interactions with components of this formula require characterization.
• Zinc (Seven Zincs + Copper) : Zinc is a cofactor of alcohol dehydrogenase, which catalyzes the oxidation of ethanol to acetaldehyde. Zinc availability directly modulates the rate of alcohol metabolism, although enzyme saturation with the cofactor occurs at relatively low concentrations, making supplementation relevant only when zinc status is suboptimal. Zinc participates in maintaining intestinal barrier integrity by influencing the expression of tight junction proteins and by functioning as a cofactor for metalloproteinases that regulate extracellular matrix renewal, complementing the effects of L-glutamine. The inclusion of copper prevents imbalances induced by zinc supplementation, since both minerals compete for absorption via the DMT1 transporter. Copper also participates in ceruloplasmin, which oxidizes iron, facilitating its transport and establishing an independent function relevant to metal homeostasis.
Stress response modulation and recovery
• L-Theanine : An amino acid present in tea that modulates neurotransmission by increasing the synthesis of GABA, the main inhibitory neurotransmitter, and by modulating glutamate receptors, thus reducing excitotoxicity. L-theanine works synergistically with dihydromyricetin, which modulates GABA-A receptors, counteracting the effects of ethanol. DHM acts during alcohol exposure, while L-theanine supports GABAergic neurotransmission during recovery. L-theanine also modulates brain waves, increasing alpha activity, which is associated with a state of relaxation without sedation, and can improve sleep quality, which is compromised during alcohol consumption by suppressing REM sleep.
• Magnesium (Eight Magnesiums) : A cofactor of over three hundred enzymes, including those involved in energy metabolism, protein and nucleic acid synthesis, and the function of ion pumps that maintain electrochemical gradients across cell membranes. Magnesium is excreted in increased amounts during alcohol consumption due to the diuretic effects of ethanol, and depletion can compromise neuromuscular function, energy metabolism, and cardiovascular homeostasis. Providing multiple forms of magnesium, including chelated forms with enhanced absorption, forms that cross the blood-brain barrier such as magnesium threonate, and sustained-release forms, ensures complete replenishment of tissue pools. Magnesium acts as an antagonist of NMDA receptors that mediate excitatory glutamatergic neurotransmission, complementing the effects of L-theanine on the GABAergic system by establishing dual modulation of neuronal excitability.
• Glycine : An amino acid that acts as an inhibitory neurotransmitter in the spinal cord and brainstem, modulating sensory information processing and motor function. It is a precursor to glutathione, along with glutamate and cysteine, establishing synergy with NACET, which provides cysteine, and pyroglutamic acid, which provides glutamate. Glycine participates in phase II conjugation in the liver by forming glycine conjugates with organic acids, facilitating excretion and contributing to detoxification, complementing its role in glutathione conjugation. Glycine also modulates glycine receptors in the central nervous system, which are distinct from the glycine site on NMDA receptors. Glycine consumption before bedtime may promote sleep quality through mechanisms that include modulation of core body temperature, which is regulated by the hypothalamus and is critical for sleep initiation.
Optimization of bioavailability and absorption
• Piperine : An alkaloid extracted from Piper nigrum that increases the bioavailability of multiple nutraceuticals by inhibiting glucuronidation, a phase II reaction that conjugates compounds with glucuronic acid, facilitating excretion; by inhibiting CYP3A4 and other cytochrome P450 enzymes that metabolize xenobiotics, reducing first-pass metabolism; and by affecting intestinal permeability, increasing absorption. Piperine can increase the bioavailability of components in this formula, including gingerols and shogaols from ginger extract, which are substrates for glucuronidation; dihydromyricetin, which is metabolized by phase II enzymes; and potentially B vitamins through effects on intestinal transporters. The modulation of absorption and metabolism pathways establishes that piperine acts as a cross-enhancing cofactor that optimizes the utilization of multiple components simultaneously. However, the timing of administration should consider that inhibition of metabolizing enzymes can also affect the metabolism of other compounds, including prescribed medications, requiring compatibility assessment when multiple substances are consumed.
What is this formula used for?
Nootropics Peru's Anti-Alcohol Protective Formula integrates a synergistic complex of dihydromyricetin, glutathione precursors, B-complex vitamin cofactors in bioactive forms, L-ornithine, and standardized ginger extract. It is designed to support the liver's metabolic capacity for processing ethanol and acetaldehyde by supporting phase I and phase II detoxification systems that catalyze the oxidation and conjugation of alcohol metabolites. The formulation promotes cellular redox homeostasis by providing NACET, a lipophilic glutathione precursor with increased bioavailability compared to standard N-acetylcysteine, and pyroglutamic acid, which participates in the gamma-glutamyl cycle, facilitating glutathione regeneration from degradation products. Vitamin cofactors, including benfotiamine, which provides thiamine pyrophosphate for acetate-metabolizing enzymes; pyridoxal-5-phosphate, which participates in amino acid transamination and neurotransmitter synthesis; and methylcobalamin, which participates in homocysteine metabolism, ensure that vitamin B-dependent enzymes function properly during the increased metabolic demand associated with alcohol metabolism. L-ornithine supports the urea cycle, which processes ammonia generated during amino acid catabolism, an increase that occurs when alcohol metabolism generates elevated NADH, which inhibits gluconeogenesis. Ginger extract modulates inflammation by inhibiting cyclooxygenase-2 and NF-κB, and promotes gastrointestinal function through effects on motility and receptors that mediate nausea. This formula is designed for preventive use before alcohol consumption, during prolonged consumption, and post-consumption to support residual alcohol processing, or as continuous hepatic metabolic support in the context of regular alcohol consumption, complementing strategies of mindful moderation, appropriate hydration, and balanced nutrition.
Can I use this formula if I only drink alcohol occasionally?
The Anti-Alcohol Protector is appropriate for both occasional use timed with specific drinking events and regular use in the context of frequent drinking. For individuals who drink alcohol occasionally, less frequently than once a week, the preventive and post-drink dosing protocol provides support during specific events without the need for continuous daily administration. Administer two to three capsules 30 to 60 minutes before starting to drink alcohol, establishing the availability of cofactors and precursors when ethanol metabolism begins, and two to three capsules before bedtime after the last drink, providing support for the processing of residual alcohol during the night when metabolism continues. This acute use protocol is effective because components including dihydromyricetin, which modulates GABA-A receptors and the activity of alcohol-metabolizing enzymes; NACET, which provides cysteine for glutathione synthesis, which is consumed during acetaldehyde conjugation; and B vitamins, which act as cofactors for metabolic enzymes, exhibit effects that develop within hours of administration rather than requiring weeks of use for established effects. However, individuals who consume alcohol occasionally but in substantial amounts when they do drink may benefit from a short-cycle, daily use program during the week prior to an anticipated drinking event to optimize tissue glutathione stores and detoxification enzyme expression. This approach, however, requires advance planning, which may not be practical for unplanned events. The advantage of occasional use is that it avoids the need for continuous daily adherence, reducing protocol cost and complexity. However, individuals who regularly consume alcohol three or more days per week may benefit more from continuous daily administration, which maintains optimized metabolic homeostasis between drinking episodes.
Will this formula allow me to drink more alcohol without consequences?
The Anti-Alcohol Protector formula provides metabolic support to the liver's capacity to process alcohol, but it does not eliminate the effects of alcohol on the central nervous system, cardiovascular function, or judgment and motor coordination, which are compromised during intoxication. The goal of supplementation is to optimize the detoxification of ethanol and acetaldehyde by providing cofactors and precursors that promote the activity of alcohol-metabolizing enzymes and to protect against oxidative stress and inflammation generated during metabolism. It does not facilitate increased consumption or eliminate the risks associated with intoxication. Regardless of supplementation, alcohol impairs cognitive function, including memory, attention, and decision-making; alters motor coordination and reaction time, increasing the risk of accidents; and causes intoxication, which can be dangerous, particularly when blood ethanol concentrations reach levels that compromise respiratory or cardiovascular function. Supplementation does not modify blood alcohol concentrations in a way that allows for safe driving or operation of machinery after alcohol consumption, and alternative transportation arrangements are necessary regardless of formula use. Additionally, excessive alcohol consumption causes cumulative damage to multiple organs, including the liver, where chronic exposure can contribute to lipid accumulation and activation of collagen-producing stellate cells; the brain, where neurotoxicity can compromise long-term cognitive function; and the gastrointestinal tract, where compromised mucosal integrity promotes inflammation, regardless of the presence or absence of acute manifestations, which can be modulated by supplementation. The appropriate approach is to integrate supplementation with conscious moderation of the amount and frequency of consumption within limits that do not overwhelm the body's detoxification capacity, establishing that supplementation complements, but does not replace, individual responsibility in decisions regarding alcohol consumption.
How long before drinking alcohol should I take the formula?
The optimal timing for preventive dosing is 30 to 60 minutes before initiating alcohol consumption, allowing sufficient time for intestinal absorption of components and distribution to tissues, including the liver, where ethanol metabolism predominantly occurs. Absorption of water-soluble components, including B vitamins, which are absorbed via specific transporters in the jejunum, occurs relatively rapidly, with increased circulating levels detectable within 30 to 45 minutes. Lipophilic components, including benfotiamine and NACET, which require emulsification by bile salts, may require 45 to 60 minutes for optimal absorption, particularly when administered with food, which slows gastric emptying but promotes bile secretion. Dihydromyricetin reaches peak plasma concentrations approximately one to two hours after oral administration, according to limited available pharmacokinetic studies, establishing that administration 60 minutes before alcohol consumption establishes appropriate circulating levels when ethanol exposure begins. If the timing of alcohol consumption is unpredictable or if the decision to consume alcohol is made spontaneously without prior planning, administration immediately before first consumption still provides support, given that alcohol metabolism continues for hours after consumption and that components absorbed during consumption will be available when metabolism is most intense. However, effectiveness may be suboptimal compared to appropriate preventive administration, since establishing tissue levels of cofactors requires time and initial alcohol metabolism may occur before optimal levels are reached. Avoid excessively early administration (more than two hours before alcohol consumption) because components with relatively short half-lives, including NACET, may be metabolized and eliminated before alcohol exposure occurs, reducing availability when metabolic demand is highest.
Can I take this formula on an empty stomach?
Administering the Anti-Alcohol Protector on an empty stomach is technically possible, given that chelated and bioactive forms of its components exhibit enhanced bioavailability that is not entirely dependent on the presence of food. However, administration with food offers advantages that promote digestive tolerance and optimize the absorption of specific components. The presence of food in the stomach buffers direct contact of concentrated components with the gastric mucosa, reducing the likelihood of mild nausea or epigastric discomfort that some individuals experience with fasted administration, particularly during initial use before adaptation has occurred. Food containing fats stimulates bile secretion, which emulsifies lipophilic components, including benfotiamine and NACET, facilitating the formation of micelles that are absorbed in the jejunum. Meanwhile, proteins in food can provide amino acids that share transporters with some components or act as signals for the secretion of digestive enzymes and gastrointestinal hormones that modulate motility. However, for preventive use where the goal is to achieve high circulating levels rapidly before alcohol exposure, administration without food or with very light food may be preferable to maximize absorption rate by establishing peak levels more quickly, although this benefit must be weighed against the increased likelihood of gastric discomfort in sensitive individuals. If nausea or discomfort is experienced with fasted administration, switch to administration with light food that includes at least a small portion of protein and fat, such as yogurt with nuts or avocado toast, which provides a matrix without being a substantial complete meal that significantly delays absorption. For post-consumption doses before bedtime, administration with at least two glasses of water is the minimum recommended regardless of the presence of food, since hydration is critical to counteract alcohol's diuretic effects, and light food may be appropriate, although appetite may be reduced during this period.
Is this formula compatible with prescription medications?
The compatibility of the Anti-Alcohol Protector with prescribed medication requires a case-by-case evaluation considering specific drug classes and potential interaction mechanisms. Formulation components may interact with medication through competition for metabolizing enzymes, particularly cytochrome P450 enzymes that metabolize multiple drugs; through modulation of transporters that mediate intestinal absorption or renal and biliary excretion of drugs; or through additive or antagonistic effects on physiological systems that are also targets of medication. Dihydromyricetin is metabolized by phase II enzymes, including glucuronosyltransferases that also metabolize multiple drugs, establishing a potential for competition that may modulate circulating levels of medication, although specific evidence of clinically significant interactions is limited. Ginger extract inhibits cyclooxygenase-2 and may modulate platelet aggregation by affecting thromboxane production, raising theoretical concerns for individuals taking anticoagulants, including warfarin, or antiplatelet agents such as clopidogrel. However, the dose of gingerols in this formula is modest, and the risk of significant interactions is likely low, requiring caution rather than an absolute contraindication. Supplemental B vitamins generally do not interact significantly with common medications, although concurrent administration with levodopa should be avoided, as pyridoxine increases peripheral decarboxylation of levodopa, reducing its arrival in the central nervous system. This necessitates a separation of at least four hours. Individuals taking multiple medications, particularly immunosuppressants, antidiabetic drugs that modulate blood glucose (where alcohol also has potential hypoglycemic effects), or thyroid medication, should communicate with their prescriber regarding supplementation, providing complete information on the composition for compatibility assessment. Maintain a time separation of at least two hours between medication and supplement as a general precaution that minimizes interactions due to competitive absorption, and monitor response to medication during the first weeks of formula use, observing if medication effectiveness is modified, suggesting an interaction that requires dose adjustment or discontinuation of the supplement.
Can I use this formula if I have compromised liver function?
Individuals with documented liver impairment, including elevated liver transaminases, impaired synthetic function evidenced by reduced albumin or coagulation factors, or clinical manifestations of liver failure, should exercise caution with supplementation and consult with a hepatologist or a healthcare professional managing liver conditions before initiating use. The liver is the primary site of metabolism for formula components, including dihydromyricetin, which is conjugated with glucuronic acid; NACET, which is hydrolyzed and metabolized; and B vitamins, which are phosphorylated and participate in multiple reactions. Therefore, impaired liver function can alter the pharmacokinetics of these components, leading to accumulation or altered metabolism. Furthermore, although the formula aims to support liver function during alcohol metabolism, established liver impairment fundamentally modifies the metabolic response to alcohol, reducing detoxification capacity and substantially increasing the risk of adverse effects. Therefore, alcohol consumption in the context of liver disease is highly inadvisable, regardless of supplementation. The individual components, including silymarin, which is commonly used in the context of impaired liver function, and N-acetylcysteine, which is clinically used in cases of acetaminophen poisoning that compromises liver function, suggest that glutathione precursors may be beneficial. However, appropriate dosage, route of administration, and monitoring in the context of established liver disease differ from its use as a preventative supplement in individuals with normal liver function. Individuals with a history of liver impairment that has fully resolved and whose liver function has normalized may consider using this formula with periodic monitoring of liver function through biochemical analyses that evaluate transaminases, bilirubin, and synthetic function, ensuring that supplementation does not produce unanticipated adverse effects. It is critical to recognize that supplementation is not a substitute for alcohol abstinence, which is a fundamental recommendation for individuals with liver impairment, and that the use of this formula should never be interpreted as facilitating safe alcohol consumption in the context of liver disease.
How many capsules should I take if I plan to drink a significant amount of alcohol?
The appropriate dosage should consider the anticipated amount of alcohol, the duration of the drinking period, and individual body mass, although it is critical to recognize that no supplementation dose can fully compensate for excessive alcohol consumption that overwhelms the liver's detoxification capacity. For moderate consumption, defined as two to four standard drinks over a two- to four-hour period, a standard protocol of two to three preventative capsules thirty to sixty minutes before consumption and two to three post-consumption capsules before bedtime is generally appropriate. For more extensive consumption anticipated to include four to six drinks over a four- to six-hour period, consider a preventative dose of three capsules, an additional dose of two capsules approximately halfway through the drinking period to maintain levels of components that are metabolized during prolonged exposure, and a post-consumption dose of three capsules before bedtime. Avoid exceeding a total dose of six to eight capsules in a 24-hour period to prevent excessive intake of individual components, particularly B vitamins. Although tolerable upper limits are high for most B vitamins, very high doses can cause manifestations, including peripheral neuropathy, with pyridoxine at doses exceeding 200 milligrams daily for months, although the risk with occasional use is low. For individuals with a high body mass index (BMI) exceeding 90 to 100 kilograms, a proportional increase in dose to the upper end of the range may be appropriate to compensate for increased volume of distribution, while individuals with a low BMI under 60 kilograms may start at the lower end of the range and assess their response. However, the emphasis should be on moderating the amount of alcohol consumed rather than increasing the supplementation dose to compensate for excessive consumption, recognizing that the liver's detoxification capacity has absolute limits that cannot be extended indefinitely through supplementation, and that cumulative alcohol damage occurs regardless of the effectiveness of supplementation in modulating acute manifestations.
Can I combine this formula with other supplements?
Combining the Alcohol Protector with other supplements is generally compatible, although it requires consideration of the composition of other supplements to prevent excessive duplication of components or interactions that may modulate absorption or effects. If you take a multivitamin containing B vitamins, check the dosages of thiamine, pyridoxine, and cobalamin in the multivitamin and add them to the Alcohol Protector content to ensure that the total intake does not exceed tolerable upper limits, although for most B vitamins these limits are high enough that excess is unlikely with typical supplementation. If you take mineral supplements, including zinc, magnesium, or selenium, which are involved in antioxidant function and detoxification, maintain a time separation of at least two hours between the Alcohol Protector and mineral supplements to minimize competition for absorption via shared divalent metal transporters. Combining supplements with complementary ingredients, including alpha-lipoic acid (which regenerates other antioxidants), silymarin (which modulates liver function), probiotics (which improve intestinal barrier integrity), or L-glutamine (which fuels enterocytes), can provide additional support through complementary mechanisms. However, the overall cost and protocol complexity should be considered when evaluating whether the benefits justify administering multiple supplements. Supplements containing stimulants, including high doses of caffeine, should be used with caution during alcohol consumption, as stimulant effects can mask the perception of intoxication levels, leading to excessive consumption without proper recognition of impaired cognitive and motor function. Avoid combining supplements with kava, valerian, or other compounds with sedative effects, as these can potentiate the depressant effects of alcohol on the central nervous system, increasing the risk of excessive sedation or respiratory depression. If you regularly take multiple supplements, consider consulting a healthcare professional for a complete protocol review, evaluating compatibility, the necessity of each component, and optimizing administration timing to minimize interactions while maximizing the effectiveness of individual components.
Will this formula affect my ability to drive after drinking?
The Anti-Alcohol Protector formula does not alter blood ethanol concentration or eliminate the effects of alcohol on cognitive function, motor coordination, reaction time, or judgment, which are critical for safe driving. Although supplementation can modulate some aspects of alcohol metabolism by supporting the activity of liver enzymes that oxidize ethanol to acetaldehyde and acetaldehyde to acetate, the rate of alcohol metabolism has a fixed limit determined by the amount of alcohol dehydrogenase in hepatocytes, which processes approximately seven to ten grams of ethanol per hour, regardless of supplementation. Alcohol compromises multiple domains of neurological function, including sustained and divided attention, which is necessary for the simultaneous monitoring of multiple aspects of the driving environment; visuomotor coordination, which determines the accuracy of motor responses to visual stimuli; and executive function, including the inhibition of inappropriate responses and decision-making under time pressure. The modulation of GABA-A receptors by dihydromyricetin, which counteracts some of the effects of ethanol on these receptors according to preclinical studies, should not be interpreted as a complete restoration of cognitive and motor function to sober levels, and evidence in humans characterizing effects on driving parameters is lacking. Regardless of supplementation, any alcohol consumption that results in a blood ethanol concentration above legal limits, which vary by jurisdiction but are typically 50 to 80 milligrams per deciliter for regular driving, constitutes impaired driving ability and poses a legal risk. The only safe approach is to plan alternative transportation before consuming alcohol, including designating a sober driver, using ride-sharing services, or remaining at the location where consumption occurs until the blood alcohol concentration returns to zero through metabolism, which requires several hours depending on the amount consumed. Supplementation should not create a false sense of confidence in the ability to drive safely after alcohol consumption, and individual responsibility for decisions about driving after drinking is not modified by the use of supplements.
Can I take this formula while pregnant or breastfeeding?
The use of alcohol-based supplements during pregnancy and breastfeeding is discouraged due to multiple considerations, including insufficient safety evidence for specific components in these populations, and fundamentally because alcohol consumption during pregnancy is contraindicated given the risk of teratogenic effects on fetal development, including neurodevelopmental impairment that can result in permanent cognitive and behavioral deficits. No level of alcohol consumption during pregnancy has been established as completely safe, and the universal recommendation from health organizations is complete abstinence from alcohol during gestation. Therefore, the use of supplements designed to support alcohol consumption is fundamentally inappropriate in the context of pregnancy. During breastfeeding, alcohol passes into breast milk, reaching concentrations similar to maternal blood levels, and infant exposure to alcohol can compromise neurodevelopment and disrupt sleep and feeding patterns. Therefore, alcohol consumption during breastfeeding is also discouraged. The individual components of the formula, including B vitamins, are essential nutrients during pregnancy and lactation. However, specific doses in supplemental formulations and combinations with other components, including dihydromyricetin, where evidence of safety in pregnancy is lacking, have not been systematically evaluated in these populations. If you are pregnant, planning a pregnancy, or breastfeeding, prioritize complete abstinence from alcohol, which is a fundamental recommendation for protecting fetal and neonatal development. If you require supplementation with B vitamins or glutathione precursors for specific indications, consult with an obstetrician or healthcare professional for recommendations on formulations specifically designed for pregnancy and lactation that have been evaluated for safety in these populations and provide appropriate doses of nutrients without components of unestablished safety.
What should I do if I experience side effects?
Tolerance to the Anti-Alcohol Protector is generally good, given that the formulation uses bioactive and chelated forms of components with improved bioavailability and superior digestive tolerance compared to simpler forms. However, some individuals may experience transient reactions, particularly during the first few days of use. If you experience mild gastric upset, nausea, or epigastric discomfort, administer the formula with substantial food containing protein and fat, which provides a matrix that cushions contact with the gastric mucosa. Alternatively, consider temporarily reducing the dose to one or two capsules daily for a week, allowing for gradual adaptation before progressing to the standard dose. Transient changes in stool consistency or frequency that may occur during the first few days, reflecting modulation of intestinal motility by ginger extract, typically resolve with continued use and do not require intervention if they are not severe. However, if diarrhea is significant or persistent beyond one week, consider reducing the dose or temporarily discontinuing the product. If you experience headache, unusual alertness, or difficulty initiating sleep, which may reflect sensitivity to specific components, consider taking the product earlier in the day, avoiding nighttime administration, which may interfere with sleep in sensitive individuals, or consider reducing the dose. Manifestations suggestive of hypersensitivity, including urticaria, generalized itching, facial or lip swelling, difficulty breathing, or wheezing, require immediate discontinuation of the formula and appropriate medical evaluation. Although allergic reactions to components of this formula are rare, they can occur in predisposed individuals. If you experience severe manifestations that compromise daily functioning, manifestations that persist beyond two weeks without improvement despite adjustments in dosage and timing, or any manifestation that causes significant concern, discontinue use and consider professional evaluation to determine the cause and compatibility of the formula with your individual conditions. Document specific manifestations, including timing of administration, severity, and duration, as this provides useful information for assessing causality and decisions regarding restarting with modifications or permanent discontinuation.
Can I take this formula if I follow a vegetarian or vegan diet?
The compatibility of the Anti-Alcohol Protector with vegetarian or vegan diets depends on the specific capsule composition, which may vary between batches or presentations. Check the ingredient panel on the product label to identify the capsule composition. Gelatin, which is derived from animal collagen, typically of bovine or porcine origin, indicates that the formulation is not appropriate for vegans and possibly not appropriate for vegetarians, depending on specific restrictions. Hydroxypropyl methylcellulose, a plant-based polymer derived from cellulose, indicates compatibility with vegetarian and vegan diets. The active ingredients in the formula, including dihydromyricetin extracted from Hovenia dulcis, ginger extract from Zingiber officinale, and L-ornithine, which is typically produced through bacterial fermentation, are appropriate for vegetarians and vegans. B-complex vitamins may be derived from synthetic or fermentation sources, which are appropriate, or in some cases from animal sources, which are not appropriate. Verification with the manufacturer is required if strict adherence to these sources is necessary. NACET, as a synthetic derivative of N-acetylcysteine, is appropriate regardless of source, while pyroglutamic acid can be derived from plant or synthetic sources. Vegetarians and vegans may have an increased risk of B-complex vitamin deficiencies, particularly vitamin B12, which is absent in unfortified plant foods, making supplementation with bioactive forms such as methylcobalamin particularly relevant in this population. If you follow a strict vegetarian or vegan diet and the capsule composition is not appropriate, consider opening the capsules and mixing the contents with food or beverages that mask the flavor, or contact the manufacturer to inquire about the availability of a vegetable capsule formulation. A well-planned plant-based diet provides multiple components that support liver function and redox homeostasis, including antioxidants from fruits and vegetables, amino acids from legumes and grains, and healthy fats from nuts and seeds, establishing that while supplementation can provide additional support, a balanced diet is fundamental for optimizing metabolic function.
Does this formula interact with coffee or caffeinated beverages?
The interaction between alcohol protectors and caffeine is complex because both compounds are metabolized by hepatic enzymes, including cytochrome P450, and because the combination of alcohol and caffeine produces effects that must be considered regardless of supplementation. Caffeine is predominantly metabolized by CYP1A2, while other components of the formula are metabolized by multiple isoforms, including CYP2E1 for some compounds. This establishes that direct competition for metabolism is limited, although modulation of enzyme expression by one compound can affect the metabolism of another. Combining alcohol with caffeine at high doses is problematic because caffeine's stimulant effects on the central nervous system can mask the perception of the level of alcohol intoxication, which has depressant effects. This allows individuals to consume larger amounts of alcohol without properly recognizing the impairment of cognitive and motor function, thus increasing the risk of severe intoxication and poor decision-making. This interaction between alcohol and caffeine occurs independently of alcohol protector use and is not significantly modulated by supplementation, making caution with simultaneous consumption of alcohol and caffeine advisable regardless. Consuming coffee or tea in moderate amounts with formula administration outside the context of alcohol consumption is generally compatible without significant interactions. However, individuals sensitive to caffeine may find that combining caffeine with components that modulate neurotransmission, including B vitamins involved in neurotransmitter synthesis, can subtly modulate its effects. If you consume caffeinated beverages during or after alcohol consumption, limit the amount to moderate levels, avoiding energy drinks with very high caffeine content. Maintain adequate hydration with water, as both alcohol and caffeine have diuretic effects that can exacerbate dehydration. Remember that the presence of caffeine does not alter blood alcohol concentration or restore the ability to drive safely after drinking.
How long do the effects last after taking the formula?
The duration of effects of alcohol protectants varies depending on the specific component, as each compound has distinct pharmacokinetics determined by absorption, distribution, metabolism, and excretion. Water-soluble B vitamins reach peak plasma concentrations within one to three hours of administration and are predominantly excreted in urine with a half-life of several hours, indicating that elevated levels persist for approximately four to eight hours after administration, although individual variability is substantial. NACET is hydrolyzed by tissue esterases, releasing N-acetylcysteine, which has a half-life of approximately two to three hours. Its effects on the provision of cysteine for glutathione synthesis are most pronounced during the first four to six hours, although glutathione synthesized from the provided cysteine persists in cells for longer periods. Dihydromyricetin has a half-life that is not fully characterized in humans, although preclinical studies suggest relatively rapid elimination within several hours. These studies indicate that its effects on GABA-A receptor modulation and alcohol metabolism are likely most pronounced during the four- to eight-hour period following administration. Gingerols and shogaols from ginger extract are metabolized by glucuronidation and sulfation, with a half-life of several hours, although metabolites may persist in circulation for longer periods. For preventive use and during alcohol consumption, the effects are most relevant during the period of active alcohol metabolism, which extends for six to eight hours after the last consumption, depending on the amount. Therefore, the timing of administration should consider that the peak effects of the components should coincide with the period of maximum metabolic demand. For use as continuous hepatic metabolic support by daily administration, cumulative effects on the expression of detoxification enzymes and replenishment of tissue glutathione stores develop over weeks and persist for days after discontinuation, establishing that sustained effects differ from acute effects of individual doses.
Can I use this formula to recover after a night of heavy drinking?
The Anti-Alcohol Protector can provide support during recovery after alcohol consumption by supplying glutathione precursors that promote the neutralization of residual reactive species, vitamin cofactors that support energy metabolism and the function of enzymes that process remaining alcohol metabolites, and ginger extract that modulates inflammation and gastrointestinal function. If you did not administer a preventative dose before consumption or a post-consumption dose immediately afterward, taking two to three capsules upon waking with plenty of water and a light meal, if tolerated, can provide support during the recovery phase. However, its effectiveness is suboptimal compared to a full protocol that includes preventative dosing, since oxidative damage and glutathione depletion have already occurred overnight when alcohol metabolism was more intense without optimal availability of cofactors and precursors. During recovery, prioritize hydration by consuming at least two to three liters of water throughout the day to compensate for dehydration caused by the diuretic effects of alcohol. Consume electrolytes through broths that provide sodium or coconut water that provides potassium. Eat a balanced diet that includes protein for liver protein regeneration, fruits and vegetables that provide dietary antioxidants, and carbohydrates for glycogen replenishment. Additional sleep, if possible, promotes recovery, as many cellular repair processes and the consolidation of metabolic homeostasis occur during sleep. However, sleep quality immediately after alcohol consumption may be compromised, requiring recovery during subsequent nights. Avoid strenuous exercise for 24 to 48 hours after significant alcohol consumption, as the metabolic demands of exercise combined with alcohol metabolism recovery can cause excessive stress. Light activity such as walking promotes circulation without placing a substantial strain on the body. Recognize that full recovery from overconsumption can take 48 to 72 hours depending on the amount consumed and individual recovery capacity, and that supplementation provides support but does not magically accelerate processes that require time for normalization of metabolic homeostasis, regeneration of depleted reserves, and repair of cellular damage that occurred during exposure.
Will this formula help with a hangover the next day?
The modulation of symptoms associated with alcohol consumption the following day, commonly referred to as a hangover, depends on multiple factors, including the amount of alcohol consumed, which determines the metabolic load; adherence to a preventive and post-consumption dosing protocol that optimizes the availability of cofactors during metabolism; appropriate hydration, which minimizes dehydration that significantly contributes to symptoms; and individual factors, including genetic polymorphisms that affect alcohol metabolism. The Anti-Alcohol Protector, by supporting the detoxification of acetaldehyde (a highly reactive metabolite that contributes to adverse symptoms), neutralizing reactive species that generate inflammation and compromise cellular function, and providing ginger extract, which modulates inflammation and gastrointestinal function, can reduce the intensity of some symptoms in some individuals. However, variability in response is substantial, and some individuals may experience pronounced benefits while others experience minimal modulation. Manifestations that may be most susceptible to modulation include nausea and gastrointestinal discomfort, where ginger extract has well-characterized effects on motility and receptors that mediate nausea, and possibly headache, where modulation of inflammation and maintenance of hydration contribute, although the mechanisms of alcohol-associated headache are complex, including vasodilation, dehydration, and effects on neurotransmission. Fatigue and cognitive impairment may be less susceptible to modulation since they partially reflect a deficit in sleep quality that cannot be fully compensated for by supplementation, and residual effects of alcohol on neurological function that persist during the recovery period. It is critical to recognize that the absence of severe manifestations or a reduction in the intensity of manifestations does not indicate that cellular damage did not occur, since many effects of alcohol on tissues, including oxidative stress, impaired protein synthesis, and altered metabolic homeostasis, occur without necessarily correlated subjective manifestations. Therefore, minimizing manifestations should not be interpreted as a license for increased consumption. The optimal approach is to use the formula as a component of a comprehensive strategy that includes moderation of consumption, proper hydration, balanced diet and adequate sleep, rather than as a sole solution for complete elimination of manifestations that allows consumption without consequences.
Do I need to cycle the use of this formula or can I take it continuously?
The optimal usage pattern depends on the frequency of alcohol consumption and individual goals. For individuals who consume alcohol occasionally, less frequently than once a week, exclusive use as a preventative and post-consumption dosage, synchronized with specific drinking events, without continuous daily administration between events, is appropriate and does not require the implementation of formal cycles since use is inherently intermittent. For individuals who consume alcohol regularly, three or more days a week, and use the formula as continuous hepatic metabolic support by administering two capsules daily, implementing cycles with periods of eight to twelve weeks of use followed by breaks of seven to ten days is recommended to evaluate sustained effects independent of active supplementation and to prevent excessive accumulation of components. However, the risk associated with components in this formula is low, given that B vitamins are water-soluble and efficiently excreted, and other components are metabolized and eliminated without substantial accumulation. Breaks allow homeostatic regulatory mechanisms that may be downregulated during continuous exogenous supplementation to return to baseline sensitivity, although evidence that this downregulation occurs with components of this formula is limited, making cycling a precaution rather than an established necessity. During breaks, maintain a balanced diet rich in glutathione precursors from dietary sources, including proteins that provide sulfur-containing amino acids, cruciferous vegetables that induce the expression of detoxification enzymes, and sources of B vitamins, and monitor parameters such as energy, digestion, and recovery after alcohol consumption to assess for dependence on supplementation. If significant manifestations return during a break, suggesting that improvements depend entirely on active supplementation, consider that prolonged continuous use may be appropriate, although assessment of alcohol moderation and optimization of independently influencing lifestyle factors should be a priority. Continuous use for years without breaks is a valid option, particularly when alcohol consumption is frequent and when perceived benefits justify continuation, although periodic monitoring with biochemical liver function tests provides objective assessment that complements subjective observation.
Can I open the capsules and mix the contents with food or drinks?
The capsules of the Anti-Alcohol Protector can be opened and the contents mixed with food or beverages if swallowing whole capsules is problematic, although this modification requires some consideration. The taste of components when released directly without encapsulation can be noticeable and potentially unpleasant, particularly given the ginger extract content, which has a pronounced spicy flavor. Mixing with strongly flavored foods or beverages that mask the taste of the components, such as yogurt, fruit smoothies, applesauce, or juice, is recommended. Consume the entire mixture immediately after preparation to ensure full dose intake and to minimize exposure of components to air, which can cause oxidation of sensitive compounds. However, the stability of most components in this formula is adequate at room temperature for short periods. Avoid mixing with very hot liquids, which can degrade temperature-sensitive components, particularly vitamins, although components in this formula are relatively stable at normal food and beverage temperatures. Mixing with acidic foods or beverages, including citrus juice or vinegar, does not compromise component stability and may facilitate the solubilization of some compounds. If capsule opening is regularly necessary due to difficulty swallowing, consider that the integrity of chelated and bioactive forms is maintained when released in the intestinal environment, regardless of whether release occurs from a capsule that disintegrates in the stomach or from a mixture with food that is consumed. Therefore, bioavailability is not significantly compromised by capsule opening. However, the timing of release may be slightly altered, since capsule disintegration in the stomach provides a relatively coordinated release of all components, while mixing with food may result in a more gradual release as food is digested. However, the impact on effectiveness is likely minimal for most individuals.
- This product is a dietary supplement designed to support metabolic capacity during alcohol processing, and should not be used as a substitute for conscious moderation of alcohol consumption or as a facilitator of excessive consumption that compromises liver, neurological, and cardiovascular function regardless of supplementation.
- Keep the container tightly closed in a cool, dry place, protected from direct sunlight, excessive humidity and heat sources, at room temperature between fifteen and twenty-five degrees Celsius to preserve the stability of bioactive components including dihydromyricetin and B complex vitamins.
- Do not consume if the safety seal is broken or shows signs of tampering, and check the expiration date printed on the container before consuming to ensure that components maintain appropriate potency.
- Keep out of reach of vulnerable people who may misuse the product without understanding its specific purpose and proper dosage protocol.
- Use during pregnancy is strongly discouraged because alcohol consumption during pregnancy is contraindicated due to the risk of teratogenic effects on fetal development, including impaired neurological development. This supplement, designed to support alcohol consumption, is fundamentally inappropriate for this population, and safety evidence for specific components, including dihydromyricetin, during pregnancy is insufficient.
- Use during breastfeeding is discouraged because alcohol passes into breast milk, reaching concentrations similar to maternal blood concentration, and infant exposure may compromise neurological development. Therefore, alcohol consumption during breastfeeding is discouraged regardless of supplementation, and the safety of formula components during breastfeeding has not been established through controlled studies.
- Individuals with documented compromised liver function, indicated by elevated transaminases, impaired synthetic function, or clinical manifestations of liver failure, should exercise extreme caution, recognizing that alcohol consumption in the context of liver disease is highly inadvisable regardless of supplementation, and that the metabolism of formula components may be altered by compromised liver function.
- Avoid use in people with a documented history of hypersensitivity or allergic reactions to formulation components including ginger extract from the Zingiberaceae family, dihydromyricetin extracted from Hovenia dulcis, or capsule excipients including gelatin or hydroxypropyl methylcellulose according to specific composition.
- People taking prescribed medication, including anticoagulants where ginger extract may theoretically interact by modulating platelet aggregation, medication metabolized by cytochrome P450 where competition for metabolism may alter drug levels, or levodopa where pyridoxine increases peripheral decarboxylation reducing effectiveness, should maintain a time separation of at least two hours and monitor medication response during the first weeks of use.
- Maintain a temporary separation of at least four hours between administration of this supplement and thyroid medications, including levothyroxine, where minerals and other components can form complexes reducing hormone absorption, compromising thyroid function control.
- Start with a conservative dose of one to two capsules during first use, assessing digestive tolerance, particularly in individuals with known gastric sensitivity or a history of intolerance to supplements, and progress to a standard dose of two to three capsules only if tolerance is appropriate without significant manifestations.
- If you experience persistent digestive symptoms including nausea, epigastric discomfort, abdominal distension, or significant changes in bowel movements beyond two weeks of use, consider dose reduction, administration with more substantial food, splitting the dose into spaced-out doses, or temporary discontinuation with gradual re-evaluation.
- Discontinue use immediately if you experience hypersensitivity manifestations including hives, generalized itching, facial or lip swelling, difficulty breathing or wheezing, which may indicate an allergic reaction requiring appropriate evaluation, although such reactions are rare with components in this formulation.
- Do not exceed a total dose of six to eight capsules in a twenty-four-hour period regardless of the amount of alcohol consumed, to prevent excessive intake of individual components, particularly B complex vitamins, where although tolerable upper limits are high, very high sustained doses can cause adverse effects.
- Avoid combining alcohol with stimulants, including high doses of caffeine or energy drinks, which mask the perception of intoxication levels through stimulant effects that counteract the depressant effects of alcohol, allowing excessive consumption without appropriate recognition of impaired cognitive and motor function, regardless of supplement use.
- Avoid combining alcohol with central nervous system depressants, including sedatives, benzodiazepines, opioids, or sedating antihistamines, as these enhance depressant effects through additive or synergistic mechanisms, increasing the risk of excessive sedation or respiratory depression.
- Recognizing that this formula does not modify blood alcohol concentration or eliminate effects on cognitive function, motor coordination, reaction time or judgment that are critical for safe vehicle operation, establishing that alternative transportation planning is necessary regardless of supplementation when alcohol has been consumed.
- Ensure proper hydration by consuming at least two to three liters of water distributed throughout the day when formula is used, particularly during and after alcohol consumption, since the diuretic effects of alcohol generate dehydration that significantly contributes to adverse manifestations and hydration optimizes the excretion of metabolites.
- Avoid using this formula as justification or facilitation of increased alcohol consumption, recognizing that cumulative alcohol damage to the liver, brain, gastrointestinal tract, and other organs occurs independently of the effectiveness of supplementation in modulating acute manifestations.
- Individuals with a history of problematic alcohol use or dependence should recognize that this formula is not appropriate as an intervention for these conditions that require specialized approaches, and that use of a supplement designed for support during consumption may be counterproductive in the context of a need for abstinence.
- Implement usage cycles of eight to twelve weeks of daily administration followed by breaks of seven to ten days when the formula is used as continuous hepatic metabolic support in the context of regular alcohol consumption, allowing evaluation of sustained effects and prevention of excessive accumulation.
- During supplementation breaks, maintain a balanced diet rich in glutathione precursors, including proteins that provide sulfur amino acids, cruciferous vegetables that induce the expression of detoxification enzymes, and dietary sources of B complex vitamins for maintenance of metabolic homeostasis.
- Prioritize appropriate quality sleep of seven to nine hours after alcohol consumption, recognizing that although alcohol may facilitate sleep onset, it compromises architecture and quality through REM sleep suppression and fragmentation, and that full recovery requires multiple nights of restorative sleep.
- Avoid strenuous exercise, including high-intensity interval training or heavy weightlifting, for 24 to 48 hours after significant alcohol consumption, limiting activity to light to moderate exercise that promotes circulation without generating excessive cardiovascular or metabolic stress.
- Documenting the amount of alcohol consumed, timing of supplement administration, and perceived response, including intensity and duration of manifestations, provides feedback for individual protocol optimization through iterative adjustments based on accumulated experience.
- If you do not observe modulation of manifestations after three to four uses with appropriate adherence to preventive and post-consumption dosing protocol, consider that individual variability in response is substantial, reflecting genetic polymorphisms and lifestyle factors, and that effectiveness may be limited in some individuals.
- Check capsule composition in the ingredients panel if you follow specific dietary restrictions, including vegetarian or vegan diets where gelatin derived from animal collagen is not appropriate, while hydroxypropyl methylcellulose, which is a vegetable polymer, is compatible.
- People who regularly consume multiple supplements, including multivitamins containing B-complex vitamins, mineral supplements, or antioxidants, should review the total composition to prevent excessive duplication of components and to assess the need for each supplement in a comprehensive protocol.
- Maintain a temporal separation of at least two hours between this supplement and high-dose mineral supplements, particularly calcium, magnesium, or zinc, to minimize competition for absorption via shared divalent metal transporters in the intestinal mucosa.
- Inform health professionals about supplementation with this product before laboratory tests that evaluate liver function, vitamin levels, or parameters related to metabolism, allowing appropriate interpretation of results considering the influence of supplementation.
- If you experience unanticipated changes in energy, sleep, digestive function, or any other parameter during use that raises concern, temporarily discontinue supplementation for causality assessment and consider whether protocol modifications or permanent discontinuation are appropriate.
- Do not use as the sole strategy for minimizing the effects of alcohol; integrate supplementation with conscious moderation of quantity and frequency of consumption, appropriate hydration by alternating water between alcoholic beverages, consumption of food before and during alcohol intake, and prioritization of sleep and recovery.
- The effects perceived may vary between individuals; this product complements the diet within a balanced lifestyle.
- Use during pregnancy is strongly discouraged, as alcohol consumption during gestation is contraindicated due to the risk of teratogenic effects on fetal development, including a spectrum of fetal alcohol disorders that compromise neurological, facial, and multi-organ development. Therefore, any supplement designed to support alcohol consumption is fundamentally inappropriate for this population. Furthermore, the safety evidence for specific components, including dihydromyricetin, during pregnancy is insufficient, with a lack of controlled human studies evaluating effects on fetal development, organogenesis, and placental function. While B vitamins are essential nutrients during pregnancy, the specific doses in supplemental formulations combined with other components have not been systematically evaluated in pregnant women.
- Use during breastfeeding is not recommended, as alcohol consumption during lactation is discouraged because ethanol passes into breast milk, reaching concentrations that approximate maternal blood levels. Infant exposure can compromise neurological development, disrupt sleep and feeding patterns, and reduce milk production by inhibiting the let-down reflex. The safety of formula components, including dihydromyricetin, NACET, and ginger extract, during breastfeeding has not been established through studies evaluating transfer into breast milk, resulting neonatal exposure, or effects on infant development. Therefore, use during breastfeeding is discouraged due to insufficient safety characterization in this vulnerable population.
- Avoid use in individuals with severely compromised liver function, including decompensated liver failure, advanced cirrhosis with portal hypertension or ascites, or acute liver failure, since the metabolism of formula components, including the conjugation of dihydromyricetin with glucuronic acid, hydrolysis and metabolism of NACET, and phosphorylation of B vitamins, occurs predominantly in the liver, and severely compromised liver function may lead to the accumulation of components or metabolites. Crucially, alcohol consumption in the context of established liver disease is contraindicated regardless of supplementation, as alcohol exacerbates liver damage, impairs regeneration, and accelerates disease progression. Therefore, the use of a supplement designed to support liver function during alcohol consumption is inappropriate in this population, where complete abstinence is the fundamental recommendation.
- Concomitant use with anticoagulants, including warfarin, acenocoumarol, or direct oral anticoagulants, including dabigatran, rivaroxaban, and apixaban, is not recommended without appropriate evaluation. This is because ginger extract contains gingerols, which inhibit cyclooxygenase, reducing thromboxane A2 synthesis, which promotes platelet aggregation. Gingerols may modulate coagulation through additional mechanisms, although evidence of clinically significant interactions with supplemental doses of ginger is limited and predominantly theoretical. The combination of alcohol with anticoagulants independently increases the risk of bleeding through effects on platelet function and coagulation factor metabolism. Therefore, additional caution is advised when alcohol is consumed by individuals on anticoagulant therapy, regardless of supplementation.
- Avoid use in individuals taking disulfiram, a drug used to discourage alcohol consumption by inhibiting aldehyde dehydrogenase, which causes severe acetaldehyde accumulation when alcohol is consumed, producing intense aversive symptoms including facial flushing, tachycardia, hypotension, and severe nausea. Although this formula contains components that promote aldehyde dehydrogenase activity and acetaldehyde conjugation, the pharmacological inhibition by disulfiram is potent and is not significantly counteracted by supplementation. Therefore, individuals undergoing disulfiram treatment should not consume alcohol regardless of supplement use, and the use of a formula designed for support during alcohol consumption is contradictory to the therapeutic objective of alcohol deterrence.
- Use is not recommended in individuals with a documented history of severe hypersensitivity or anaphylactic reactions to plants of the Zingiberaceae family, which includes ginger, turmeric, and cardamom, since ginger extract is a component of the formulation and exposure may trigger a type I IgE-mediated hypersensitivity reaction that can manifest as urticaria, angioedema, bronchospasm, or, in severe cases, anaphylactic shock. Additionally, avoid use in individuals with known hypersensitivity to Hovenia dulcis, a source of dihydromyricetin, or to capsule excipients, including collagen-derived gelatin, which may cause reactions in individuals with allergies to specific animal proteins.
- Avoid use in individuals taking monoamine oxidase inhibitors, including phenelzine, tranylcypromine, or selegiline, at non-selective doses. Tyramine, which may be present in small amounts in herbal extracts or formed during amino acid metabolism, can trigger a hypertensive crisis in the presence of MAO inhibition, which prevents tyramine degradation. Additionally, alcohol consumption in combination with MAO inhibitors can lead to complex interactions, including potentiation of central nervous system depressant effects and modulation of neurotransmitter metabolism. Therefore, this combination requires extreme caution, regardless of supplementation.
- Use is not recommended in individuals with coagulation disorders, including hemophilia, von Willebrand disease, or severe thrombocytopenia, as ginger extract can modulate platelet function by inhibiting thromboxane synthesis, and alcohol also compromises hemostasis through multiple mechanisms, including effects on hepatic synthesis of coagulation factors and platelet function. The combination of multiple factors that compromise coagulation can increase the risk of bleeding, particularly during trauma or surgical procedures. Therefore, individuals with coagulation disorders should avoid both alcohol consumption and supplementation with components that modulate hemostasis.
- Avoid use in patients scheduled for elective surgery during the two weeks prior to the procedure, as ginger extract may modulate platelet aggregation and hemostasis, increasing the risk of perioperative bleeding, and as the metabolism of anesthetics and other drugs used during surgery may be modulated by components of the formula through effects on cytochrome P450. Additionally, alcohol consumption should be discontinued during the preoperative period to optimize liver function, reduce the risk of anesthetic complications, and facilitate postoperative healing, establishing that the use of a formula designed for support during alcohol consumption is inappropriate during the perioperative period.
- Use is not recommended in individuals with active peptic ulcers or acute gastrointestinal bleeding, as ginger extract can increase gastric secretion and modulate motility, potentially exacerbating symptoms. Furthermore, alcohol is a direct irritant to the gastric mucosa, compromising the integrity of the mucosal barrier, increasing acid secretion, and reducing the production of protective prostaglandins. The combination of alcohol consumption with an active peptic ulcer significantly increases the risk of bleeding and perforation, regardless of supplementation. Therefore, abstaining from alcohol is a fundamental recommendation during the ulcer healing period.
- Avoid use in individuals with gallstones or bile duct obstruction, as components including ginger extract may stimulate gallbladder contraction and bile flow, which in the presence of obstruction can lead to severe biliary colic or, in extreme cases, cholecystitis. NACET and other lipophilic components require emulsification by bile salts for proper absorption, establishing an increased demand for biliary function, and compromised bile flow may alter the pharmacokinetics of components, reducing absorption.
- Use is not recommended in individuals with recurrent hypoglycemia or those using antidiabetic medication, including insulin or sulfonylureas, since alcohol consumption inhibits hepatic gluconeogenesis by generating high levels of NADH, which favors the conversion of pyruvate to lactate rather than oxidation to acetyl-CoA, predisposing to hypoglycemia, particularly when alcohol consumption occurs without adequate carbohydrate intake. Although this formula contains cofactors involved in carbohydrate metabolism, it does not prevent alcohol-induced hypoglycemia. Therefore, individuals at risk of hypoglycemia should extremely moderate their alcohol consumption, monitor their blood glucose appropriately, and ensure carbohydrate intake before and during alcohol consumption, regardless of supplementation.
Let customers speak for us
from 107 reviewsLuego se 21 días sin ver a mi esposo por temas de viaje lo encontré más recuperado y con un peso saludable y lleno de vida pese a su condición de Parkinson!
Empezó a tomar el azul de metileno y
ha mejorado SIGNIFICATIVAMENTE
Ya no hay tantos temblores tiene más equilibrio, buen tono de piel y su energía y estado de ánimo son los óptimos.
Gracias por tan buen producto!
Empezé con la dosis muy baja de 0.5mg por semana y tuve un poco de nauseas por un par de días. A pesar de la dosis tan baja, ya percibo algun efecto. Me ha bajado el hambre particularmente los antojos por chatarra. Pienso seguir con el protocolo incrementando la dosis cada 4 semanas.

Debido a que tengo algunos traumas con el sexo, me cohibia con mi pareja y no lograba disfrutar plenamente, me frustraba mucho...Probé con este producto por curiosidad, pero es increíble!! Realmente me libero mucho y fue la primera toma, me encantó, cumplió con la descripción 🌟🌟🌟

Super efectivo el producto, se nota la buena calidad. Lo use para tratar virus y el efecto fue casi inmediato. 100%Recomendable.

Desde hace algunos años atrás empecé a perder cabello, inicié una serie de tratamientos tanto tópicos como sistémicos, pero no me hicieron efecto, pero, desde que tomé el tripéptido de cobre noté una diferencia, llamémosla, milagrosa, ya no pierdo cabello y siento que las raíces están fuertes. Definitivamente recomiendo este producto.

Muy buena calidad y no da dolor de cabeza si tomas dosis altas (2.4g) como los de la farmacia, muy bueno! recomendado

Un producto maravilloso, mis padres y yo lo tomamos. Super recomendado!

Muy buen producto, efectivo. Los productos tienen muy buenas sinergias. Recomendable. Buena atención.

Este producto me ha sorprendido, yo tengo problemas para conciliar el sueño, debido a malos hábitos, al consumir 1 capsula note los efectos en menos de 1hora, claro eso depende mucho de cada organismo, no es necesario consumirlo todos los días en mi caso porque basta una capsula para regular el sueño, dije que tengo problemas para conciliar porque me falta eliminar esos habitos como utilizar el celular antes de dormir, pero el producto ayuda bastante para conciliar el sueño 5/5, lo recomiendo.

Con respecto a la atención que brinda la página es 5 de 5, estoy satisfecho porque vino en buenas condiciones y añadió un regalo, sobre la eficacia del producto aún no puedo decir algo en específico porque todavía no lo consumo.

Compre el Retrauide para reducir mi grasa corporal para rendimiento deportivo, realmente funciona, y mas que ayudarme a bajar de peso, me gusto que mejoro mi relacion con la comida, no solo fue una reduccion en el apetito, sino que directamente la comida "chatarra" no me llama la atencion como la hacia antes. Feliz con la compra.

Pedí enzimas digestivas y melón amargo, el proceso de envío fué seguro y profesional. El producto estaba muy bien protegido y lo recogí sin inconvenientes.
Estoy familiarizado con los nootrópicos hace algunos años, habiéndolos descubierto en EEUU a travez de ingenieros de software. Cada protocolo es distinto, cada organismo también y la meta de uno puede ser cognitiva, por salud, por prevención, etc... Nootrópicos Perú es una tienda que brinda la misma calidad y atención al cliente, que darían en una "boutique" de nootrópicos en San José, Silicon Valley; extremadamente profesionales, atención personalizada que raramente se encuentra en Perú, insumos top.
No es la típica tienda a la que la mayoría de peruanos estamos acostumbrados, ni lo que se consigue por mercadolibre... Se detallan muy bien una multiplicidad de protocolos con diferentes enfoques y pondría en la reseña 6/5, de ser posible. Lo único que recomiendo a todos los que utilicen nootrópicos: Es ideal coordinar con un doctor en paralelo, internista/funcional de ser posible, para hacerse paneles de sangre y medir la reacción del cuerpo de cada quién. Todos somos diferentes en nuestra composición bioquímica, si bien son suplementos altamente efectivos, no son juegos y uno debe tomárselo seriamente.
Reitero, no he leído toda la información que la web ofrece, la cual es vasta y de lo poco que he leído acierta al 100% y considera muchísimos aspectos de manera super profesional e informada al día. Es simplemente una recomendación en función a mi propia experiencia y la de otros conocidos míos que los utilizan (tanto en Perú, como en el extranjero).
6 puntos de 5.
Hace un tiempo decidí probar la semaglutida y descubrí esta página. Ha sido una experiencia muy positiva: todo es claro, confiable y seguro. Mi esposa, mi hermana y yo seguimos el tratamiento, y poco a poco hemos bajado de peso y encontrado un mejor equilibrio en nuestra salud y bienestar.
Este producto funciona de manera efectiva, yo lo tomo un rato después de la última bebida, creeme que afectará de manera positiva la forma en como te sientes al día siguiente. En resumidas cuentas, esta fórmula trabaja activamente para descomponer y eliminar el acetaldehído del cuerpo, te sentirás mejor después de una noche de tragos, gran creación, gracias
Nada de resaca como lo prometen, me pareció increíble
⚖️ DISCLAIMER
The information presented on this page is for educational, informational and general guidance purposes only regarding nutrition, wellness and biooptimization.
The products mentioned are not intended to diagnose, treat, cure or prevent any disease, and should not be considered as a substitute for professional medical evaluation or advice from a qualified health professional.
The protocols, combinations, and recommendations described are based on published scientific research, international nutritional literature, and the experiences of users and wellness professionals, but they do not constitute medical advice. Every body is different, so the response to supplements may vary depending on individual factors such as age, lifestyle, diet, metabolism, and overall physiological state.
Nootropics Peru acts solely as a supplier of nutritional supplements and research compounds that are freely available in the country and meet international standards of purity and quality. These products are marketed for complementary use within a healthy lifestyle and are the responsibility of the consumer.
Before starting any protocol or incorporating new supplements, it is recommended to consult a health or nutrition professional to determine the appropriateness and dosage in each case.
The use of the information contained on this site is the sole responsibility of the user.
In accordance with current regulations from the Ministry of Health and DIGESA, all products are offered as over-the-counter food supplements or nutritional compounds, with no pharmacological or medicinal properties. The descriptions provided refer to their composition, origin, and possible physiological functions, without attributing any therapeutic, preventative, or curative properties.











